reactivity of metals
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
What is the reactivity of metals
How easily metals form these positive ions . Metals that form ions more easily are most reactive
What metals are in reactivity series list the order
-potassium
-sodium
-lithium
-calcium
-magnesium
-carbon
-zinc
-iron
-hydrogen
-copper
In the reactivity series what metals are most-least reactive (what types of metals )
most reactive metals are group 1 elements
Then group 2
And the least reactive metals are the transition metals
What equation shows the reaction of metals with acids
Acid + metal —> salt + hydrogen
How can you react metals to see how reactive they are
React each metal with acid or water to compare reactivity(how fast or violent reactions are)
Describe the reactivity of metals in acids
To see how reactive a metal is you can monitor the rate of hydrogen produced when they react with an acid.
The more reactive the metal the faster the reaction will go. The speed of the reaction is indicated by the rate at which the bubbles of hydrogen are given off a speedy reaction is shown by bubble is being pretty rapidly.
– Very reactive metals like potassium react explosively but less reactive metals such as magnesium react less violently and copper won’t react with cold dilute acids.
How can you detect the production of hydrogen
Using the burning splint test.
This involves putting a lit splint at the mouth of the tube containing the metal and acid if hydrogen is there you’ll hear a squeaky pop the more reactive the metal the more hydrogen produce produced in a certain amount of time and the louder the squeaky pop
What is the equation for the reaction of metals with water
Metal + water —> metal hydroxide + hydrogen
How can you investigate the reactivity of metals using temperature change
More reactive metals will lead to larger temperature changes.
React each of the metals with either water or acid over a set period of time.
Ensure that the surface area and the mass of the metal is the same each time.
Measure the rise in temperature.
The greater the temperature change, the more reactive the metal must be.
How do metals react with water
Some metals form positive ions when they react with water so the reaction can be used to compare their reactivity. The reaction at room temperature produces a metal hydroxide and hydrogen gas.
– Metals that are very reactive such as zinc iron and copper won’t react with water however more reactive metals such as group one metals potassium sodium as well as group 2 metals will react.
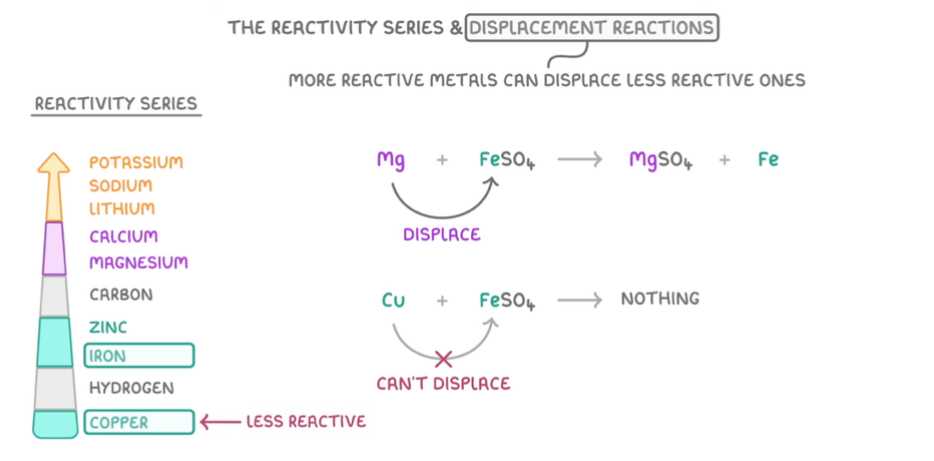
What is a displacement reaction
When more reactive metals can displace less reactive ones
Give symbol displacement reaction for magnesium and iron sulphate and copper and iron sulphate
Mg + FeSO4 —> MgSO4 + Fe
Cu + FeSO4 —> nothing happens as copper is less reactive