3.4 - energy levels in atoms
1/62
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
63 Terms
why are the electrons ‘trapped’ inside the nucleus?
because of the electrostatic force of attraction from the positively charged nucleus
does the energy of an electron in a shell fluctuate?
no, it is constant
how many electrons can each shell hold?
innermost shell - 2
every other shell - 8
which shell has the most energy?
the further from the nucleus, the more energy a shell has
what does the energy of the electron depend on?
how close it’s shell is to the nucleus
why does an electron have more energy in outermost shells? CLARIFICATION
higher potential energy
experimentally, we know that electrons release energy when de-exciting and requires an energy input when exciting
the further from the nucleus, the less effect the oppositely charged nucleus has on the electron, ergo increasing the electron’s electrostatic and potential energy. the electrons in the innermost shells also shield the outer electron from some of the effects from the nucleus
what kind of energy is the energy in an orbiting electron?
kinetic
potential
electrostatic
do all atoms have the same number of electrons?
no you stupid bitch


what is ground state?
the lowest energy state of an atom
what is the excited state?
when an atom in the ground state absorbs energy and one of it’s electrons moves to a shell at a higher energy level
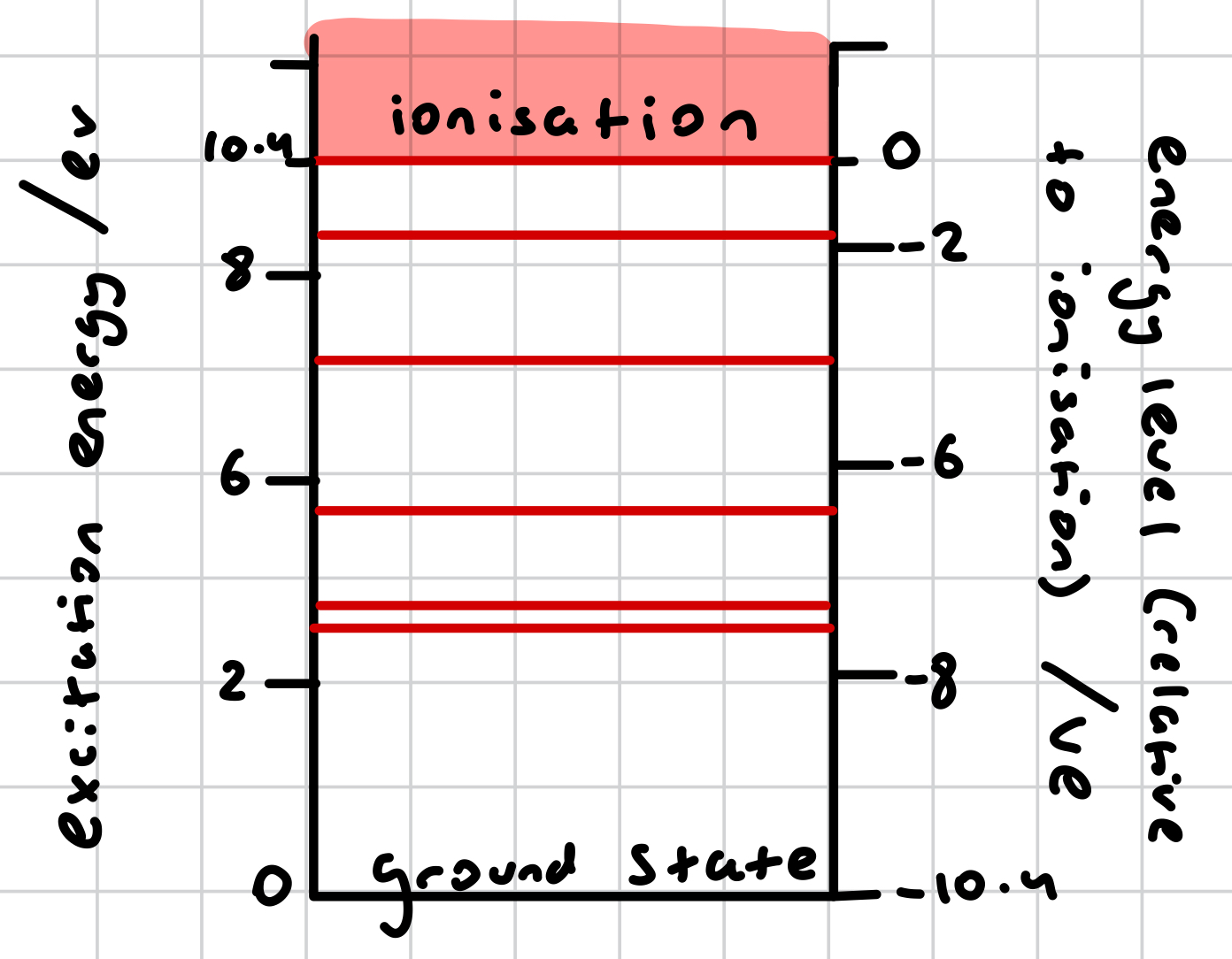
energy level diagram
shows allowed energy values of an atom
each allowed energy corresponds to a certain electron configuration within the atom
ionisation level may be considered as the zero-reference level rather than the ground state; the energy levels below ionisation level would just need to be shown as negative
what does each allowed energy level in an energy level diagram correspond to?
a certain electron configuration within the atom
what is the zero—reference level in an energy level diagram?-
ground state or ionisation energy (or both)
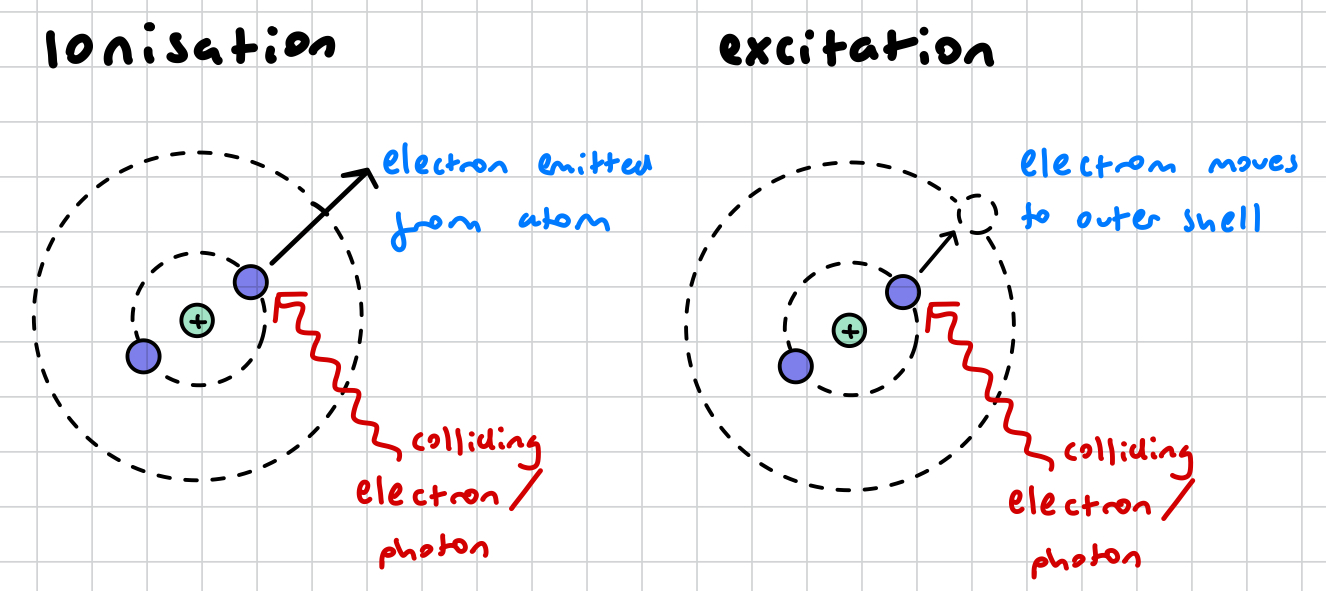
what is excitation? AND RADIATION?
electrons in an innermost shell absorb energy from a colliding electron (/ alpha beta gamma?) , moving it to an outermost shell of higher WITHOUT having enough energy to ionise it
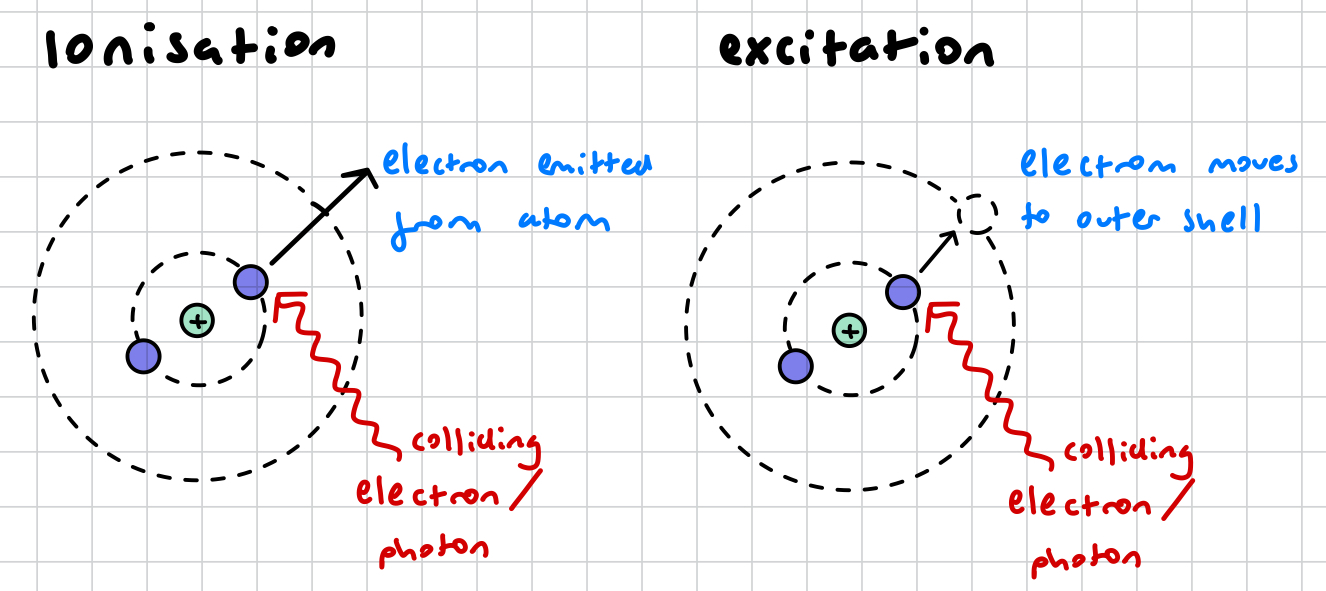
what is ionisation? AND RADIATION?
an electron in an atom’s shell absorbs the energy from a colliding electron (/ alpha beta gamma radiation?) and is knocked out of the atom
what is the difference between ionisation and excitation?
in ionisation, the electron absorbs enough energy to completely eject it form the atom. in excitation, the electron only absorbs enough energy to move it to a shell of higher energy level
in ionisation, the atom becomes unstable as it is now an ion and has a charge. in excitation, the atom has a stable charge, however it is still unstable because it has a vacancy in an innermost shell
what do gases at low pressure do when they are made to conduct electricity?
emit light
why do gases at low pressure emit light when made to conduct electricity?
atoms absorb energy as a result of excitation by collision (from the electrons in the electricity)
but they do not retain the absorbed energy permanently
the atom is unstable due to a vacancy in an inner shell
so an electron from an outermost shell moves down an energy level to fill this vacancy, emitting a gamma photon in order to lower it’s energy level (de-excitation)
this gamma photon is the light emitting
why do gases in a tube need to be low pressure when conducted to emit light?
ASKKK
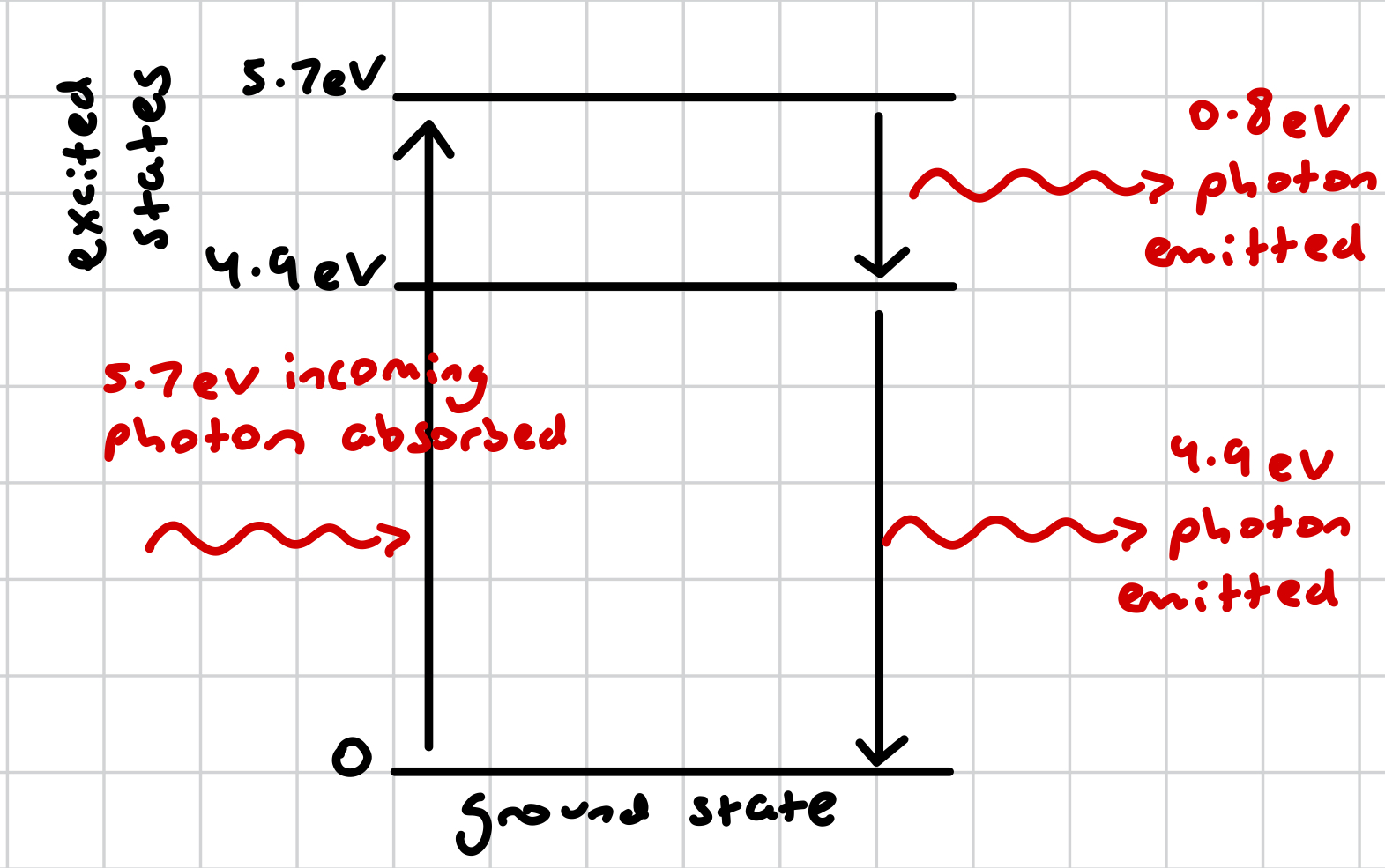
what is de-excitation (by photon emission)?
an electron in an outermost shell emits a gamma photon, releasing energy, in order to move to an innermost shell of lower energy level to fill a vacancy left by an excited electron that has now rendered the atom unstable
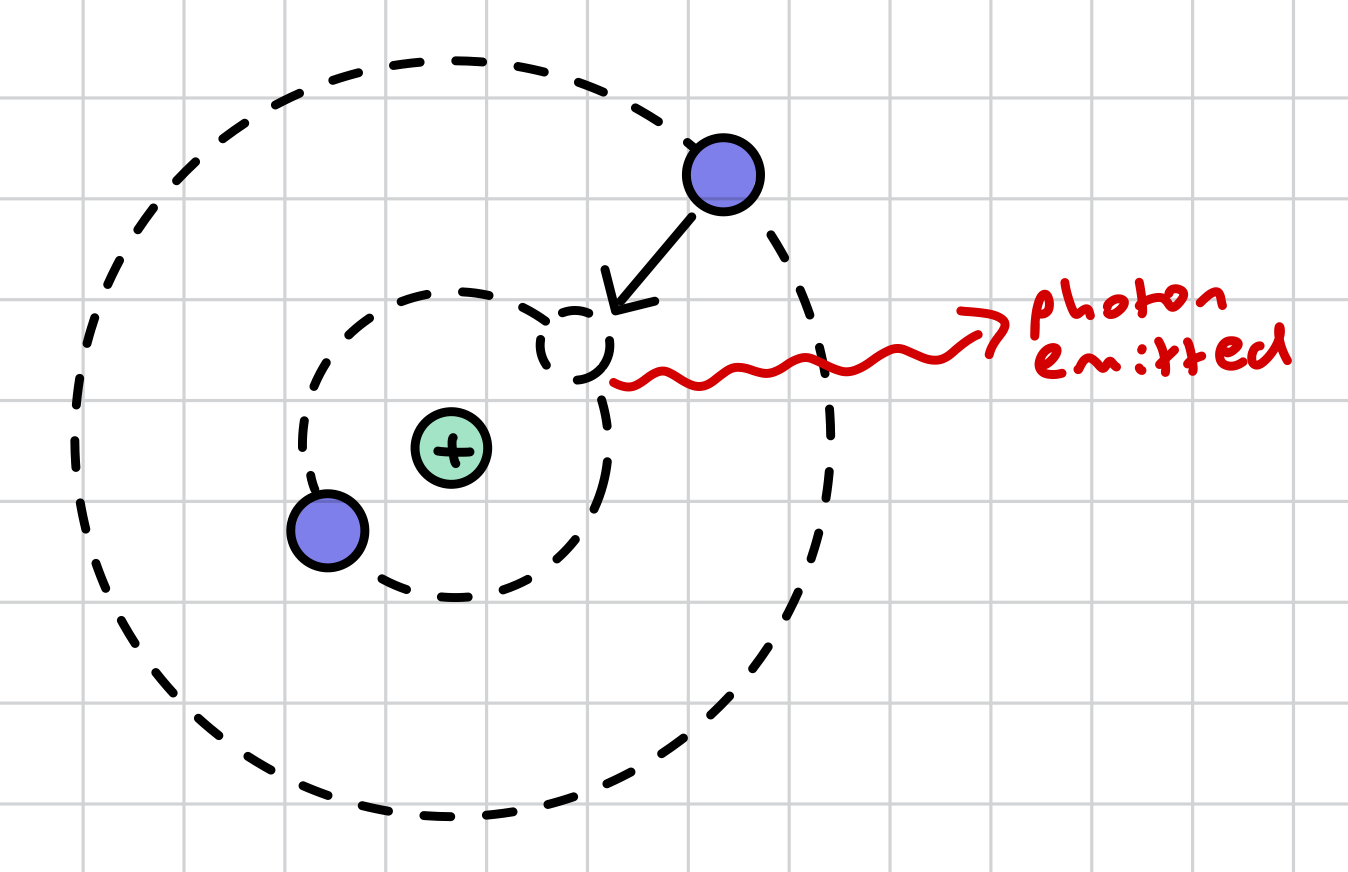
in de-excitation, what is the energy of the photon equal to?
the energy lost by the electron (when moving down an energy level, due to conservation of energy), therefore the energy lost by the atom
how can an atom de-excite?
directly, by firing a photon of energy equal to the energy the electron requires to move to a shell of lower energy level
indirectly, via several energy levels if intermediate energy levels are present
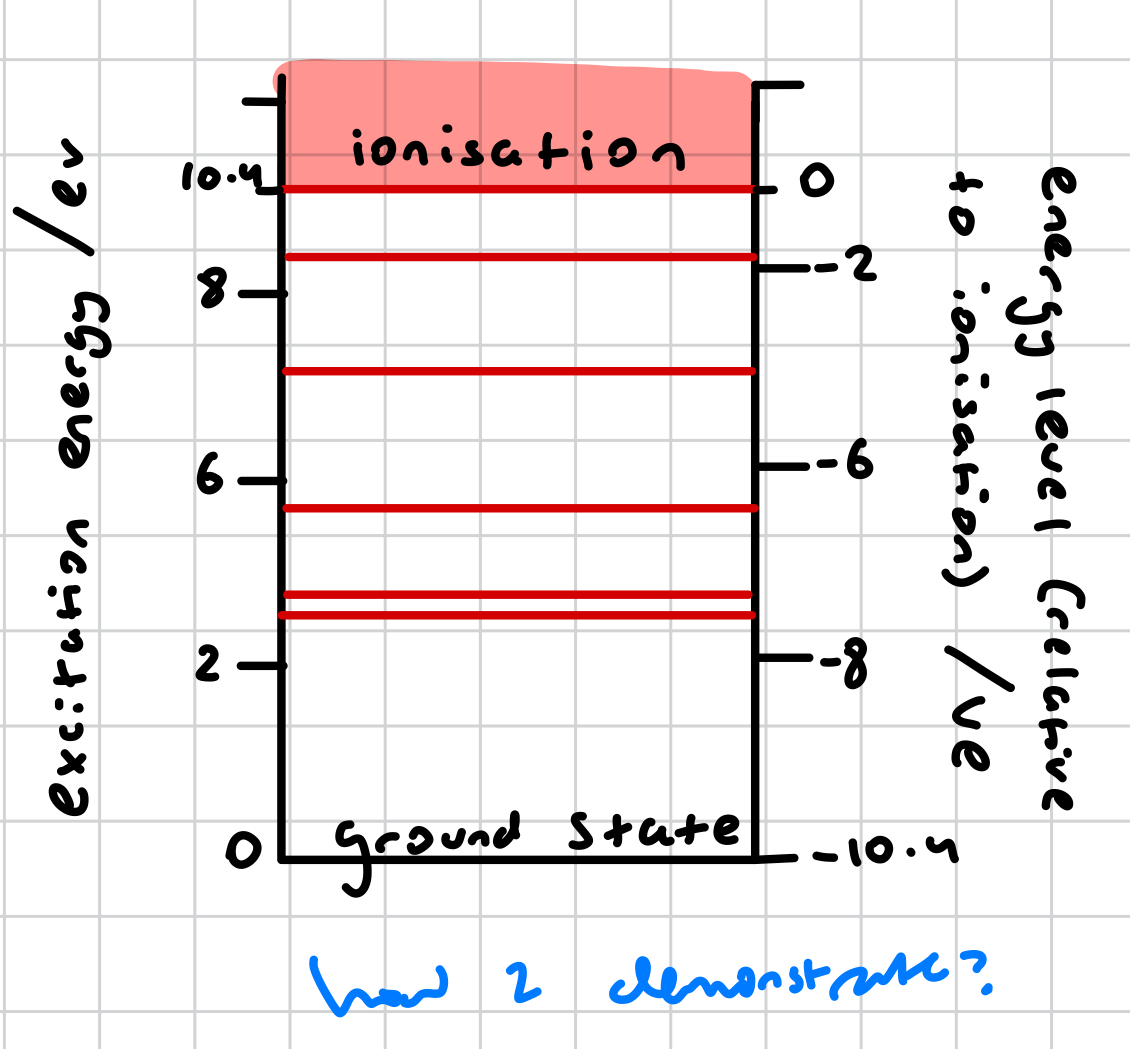
HOW TO DEMONSTRATE ON DIAGRAM?
energy of emitted photon equation
hf = E1 - E2
hf - energy of photon
h - planck’s constant, IDK IT
f - frequency (of photon)
E1 - energy level of outermost shell
E2 - lower energy level of innermost shell




what is excitation by photon?
an electron in an innermost shell can absorb a photon and move up an energy level (where there is a vacancy), providing the energy of the photon is exactly the energy difference between the 2 energy levels, otherwise the electron will not absorb the photon
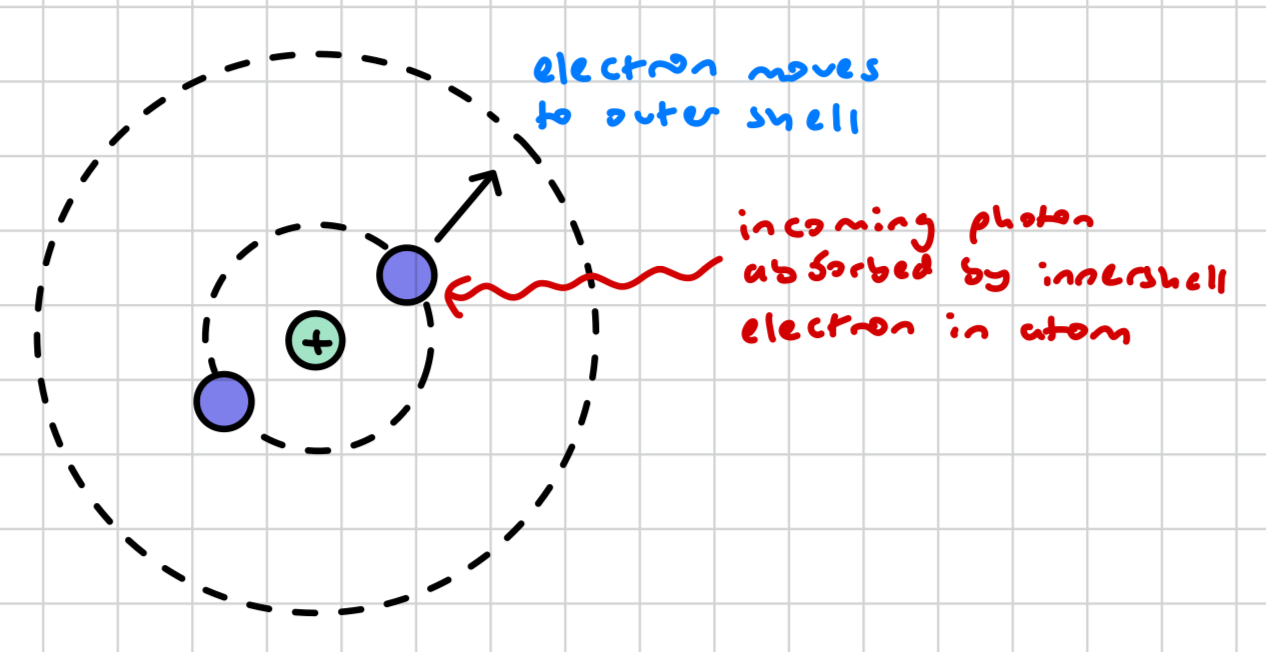
what happens in excitation by photon when the photon’s energy level is not exactly the same as the difference between 2 energy levels?
the electron will not absorb it
what is the difference between ionisation by photon and excitation by photon?
an electron in an atom will ionise if it absorbs a photon of energy level (providing it is at least the energy level for ionisation), however an excited electron requires a photon of a specific energy
when an atom de-excites, what energy level does it de-excite to?
the ground state
does the way an atom de-excites depend on the way it was excited?
no, it can de-excite directly or indirectly regardless of how it was excited
what colour light does a neon tube (filled with gas) emit when it conducts electricity?
red-orange
what is fluoresce?
substances that glow with visible light when excited by absorbing ultraviolet radiation (photons), and de-excite by emitting visible photons
what causes a substance to be fluorescent?
a source of ultraviolet radiation
what do substances absorb to fluoresce?
ultraviolet radiation as photons
what do substances emit to fluoresce?
visible photons
what happens when the source of ultraviolet radiation is removed?
the substance stops glowing
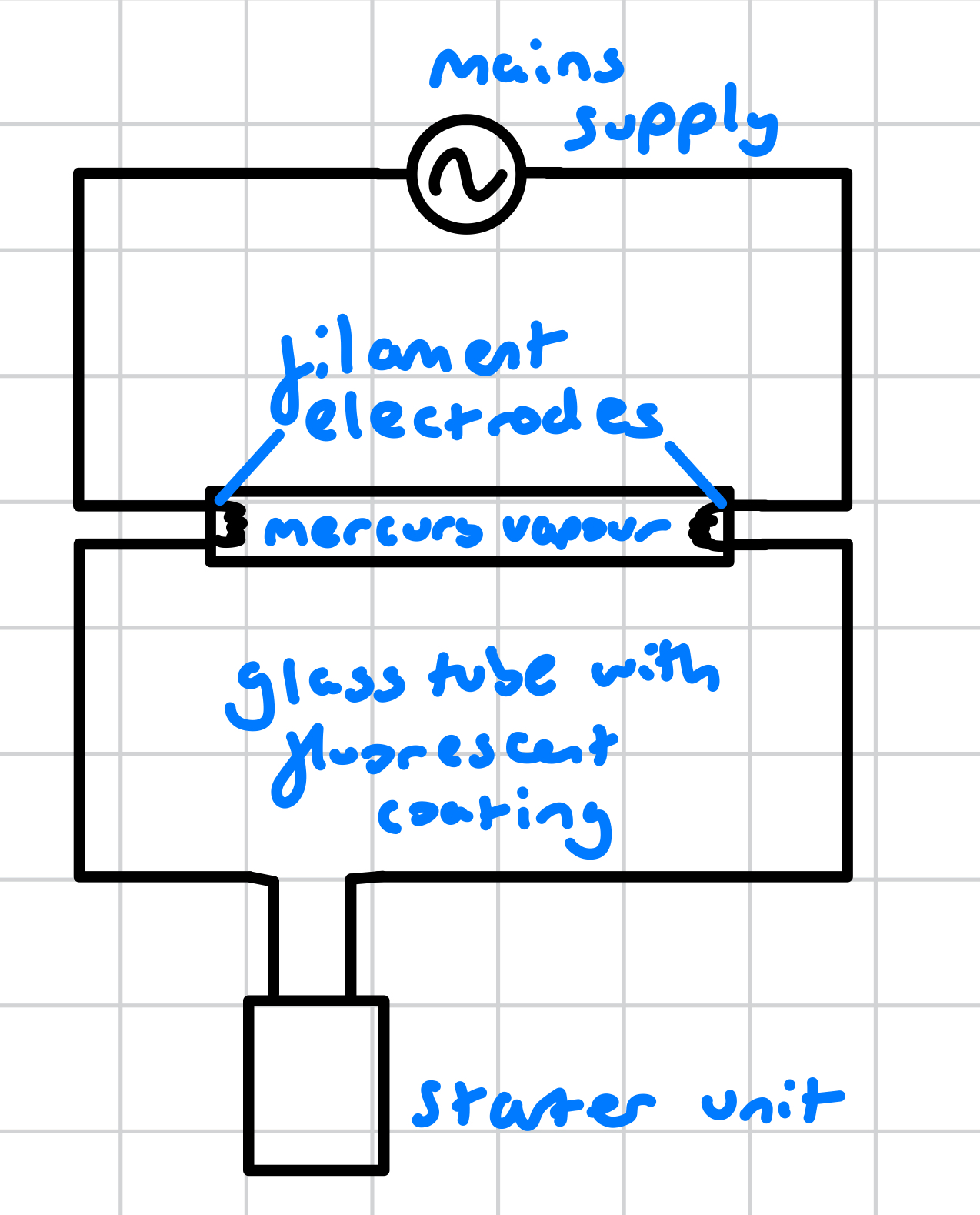
what is a fluorescent tube?
glass tube
fluorescent coating on it’s inner surface
contains mercury vapour at low pressure
emits visible light when the tube is on (i.e., conducting electricity)
what material is a fluorescent tube made of?
glass
what is inside a fluorescent tube?
fluorescent coating on it’s inner surface
mercury vapour at low pressure
what kind of coating is inside a fluorescent tube?
fluorescent coating … jeez, probably could’ve worked that one out right?
what kind of vapour is in a fluorescent tube?
mercury vapour at low pressure
why is the mercury vapour in a fluorescent tube low pressure?
IDK ASK
why does a fluorescent tube emit visible light?
ionisation and excitation occur due mercury atoms colliding with each other and the electrons in the tube
mercury atoms de-excite. they emit ultraviolet photons, as well as visible photons and photons of much less energy
the ultraviolet photons are absorbed by the atoms of fluorescent coating, causing excitation of those atoms
the coating atoms de-excite in steps, emitting visible photons
what causes excitation and ionisation in a fluorescent tube?
the mercury atoms inside the tube colliding with each other and the electrons from the electricity
what kind of photons do mercury atoms emit when de-exciting?
ultraviolet
visible
photons of much less energy
what happens to the ultraviolet photons emitted by the de-excited mercury atoms in a fluorescent tube?
they’re absorbed by the atoms of the fluorescent coating, exciting them
how does the fluorescent coating excite?
by absorbing the ultraviolet photons emitted by the de-exciting mercury atoms
how does the fluorescent coating de-excite?
in steps, emitting visible photons
what does the fluorescent coating emit when de-exciting?
visible photons
what is the visible light emitting from a fluorescent tube due to?
the emission of visible photons from the de-excitation of mercury atoms and atoms of the fluorescent coating in the tube
which is more efficient, a fluorescent tube or a filament lamp? why?
the fluorescent tube -
typical 100 W filament lamp releases 10-15 W light energy, the rest wasted as heat
fluorescent tube produces same light output with no more than a few watts wasted as heat
what is in a fluorscent tube circuit?
mains supply
filament electrodes
mercury vapour inside a glass tube with fluorescent inner coating
starter unit
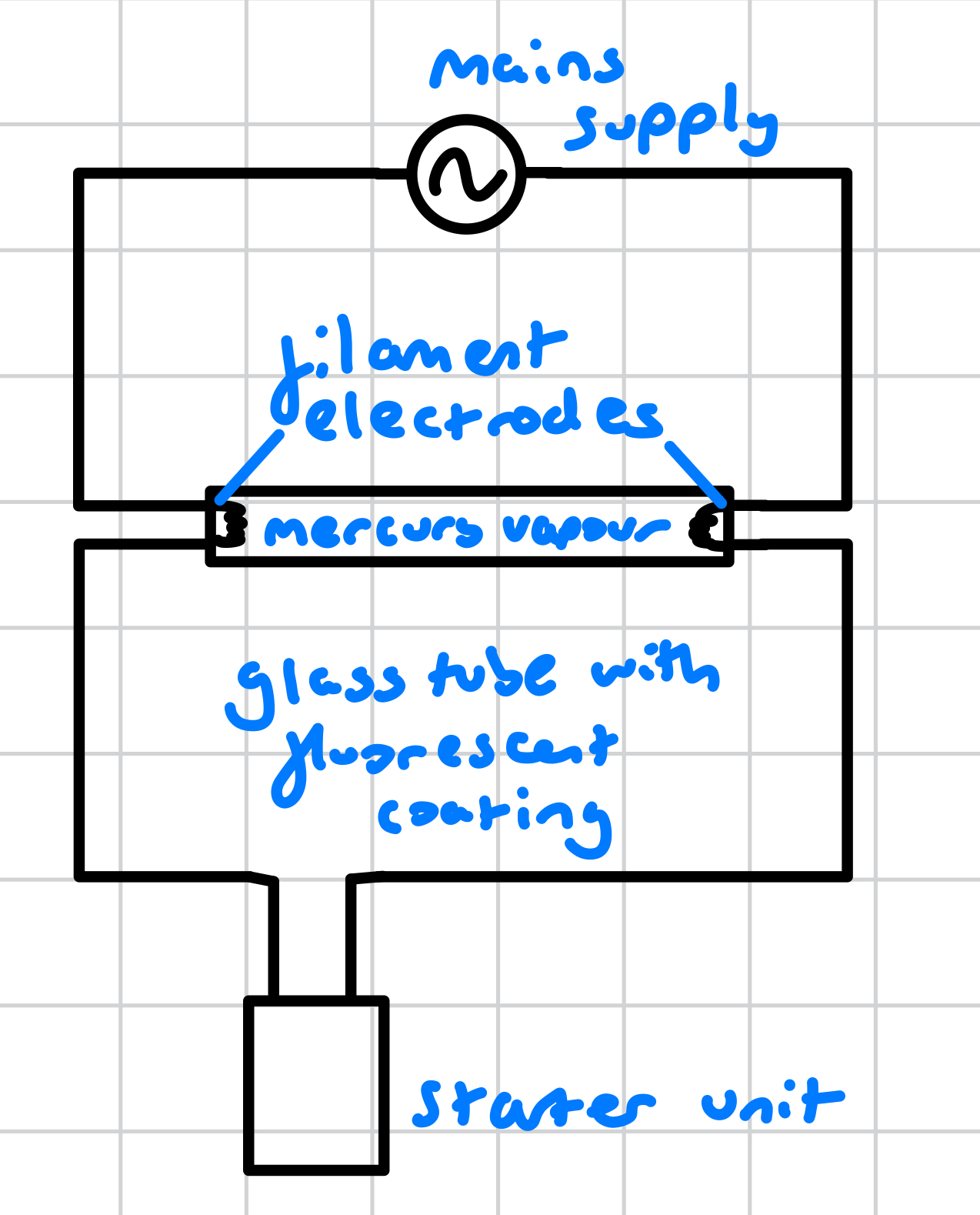
why does a fluorescent tube need a starter unit?
because the mains voltage is too small to ionise the vapour in the tube when the electrodes are cold, the starter unit heats the electrodes
how does the starter unit heat the filament electrodes?
tube switched on
argon gas in the starter unit conducts and heats a bimetallic switch, making it bend and therefore closing the switch
the current in the starter unit increases enough to heat the filament electrodes
when the bimetallic strip closes, the gas in the starter unit stops conducting
the bimetallic strip cools and the switch opens
the mains voltage now acts between the 2 electrodes, which are now hot enough for ionisation of the gas to occur
what does a fluorescent tube have on each end?
a filament electrode
what kind of gas is in the starter unit?
argon
why is argon gas used in the starter unit?
IDK ASK
what does the starter unit heat?
a bimetallic switch, and in turn the filament electrodes
what does the bimetallic switch do?
heats as the starter unit conducts, eventually bending and closing the switch so the starter unit is heating the filament electrodes instead
LOOJK INTO HOW TO START A FLUORESCENT TUBE IDK