Unit 1 Review Quiz
1/30
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
Which list of components characterizes RNA?
a phosphate group, ribose, and uracil
If you are careful, it is possible to float a pin or even a paper clip on the surface of water. This can be explained by
surface tension that is the result of the cohesive properties of water
Which of the following statements regarding carbon is false?
Carbon has the capacity to form polar covalent bonds with hydrogen
Because of the unique properties of after associated with hydrogen bonding, water evaporates from pores on the leaves of plants and draws water molecules in a continuous chain from the roots up through the vascular tissues of plants. Which of these groups of terms describes the process and properties of water that explains this?
cohesion, adhesion, and transpiration
The major class of biological molecules that are not polymers
Lipids
Linkages between monomers of proteins
Peptide Bonds
A secondary structure of proteins
Alpha Helix
A structural carbohydrate found in plants
Cellulose
Hydrolysis is involved in which of the following?
The digestion of sucrose, a disaccharide to its monomers of glucose and fructose
The process by which protein conformation is lost or broken down is
denaturation
An organic compound that is composed of carbon, hydrogen, and oxygen in a 1:2:1 ratio is known as a
carbohydrate
Which of the macromolecules below could form structural parts of the cell, be enzymes, or be involved in cell movement or communication?
proteins
The plasma membrane is composed of several different macromolecules. Which macromolecule serves as the fluid interface between the intracellular and extracellular environments?
Phospholipids
The partial negative charge at one end of a water molecule is attracted to a partial positive charge of another water molecule. What is this type of attraction called?
A hydrogen bond
Polymers of carbohydrates and proteins are all synthesized from monomers by which of the following processes?
Dehydration reactions
If the pH of a solution is decreased from 7 to 6, it means that the concentration of
H+ has increased to 10 times what it was at pH 7
Recall the structure of a typical amino acid and how the molecule has two distinct “ends.” Which two functional groups are always found in amino acids?
carboxyl and amine
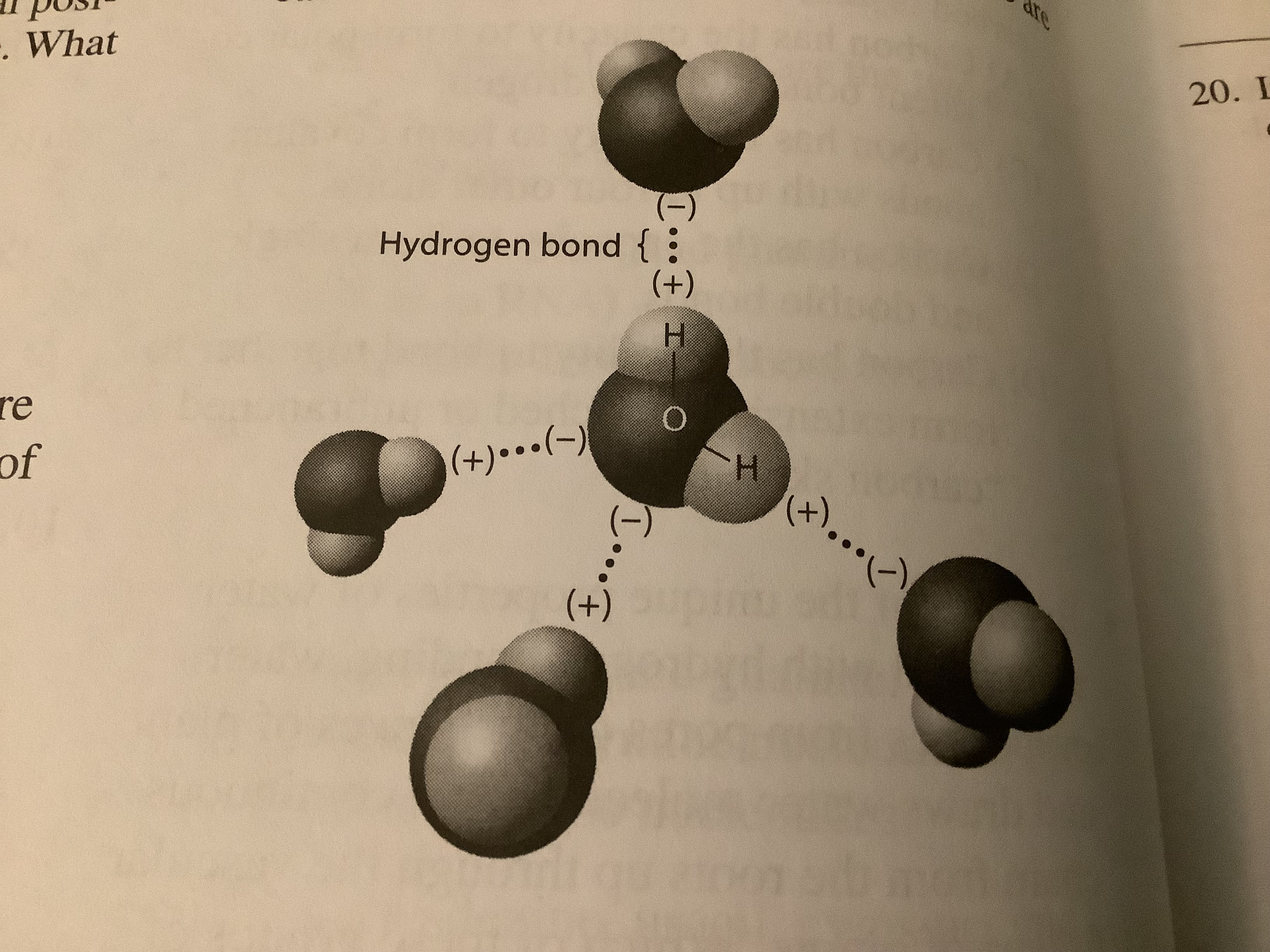
The hydrogen bonds shown in this figure are each between
an oxygen and hydrogen atom of different water molecules
The tremendous variation and unique properties of proteins are most likely a result of
interactions between R groups of the amino acids
If the dipeptide in the figure were to be digested, how would it be reduced to amino acids?
through a hydrolysis reaction in which water is added
A globular blood protein that transports vitamin A is 127 amino acids long. How many water molecules were produced in the synthesis of the protein, and by what chemical process were the amino acids joined together?
126; dehydration
Which of the biological macromolecules requires phosphorus?
nucleic acids and certain lipids
Which statement about unsaturated fats is true?
They have double bonds in their fatty acid chains
You’re the manager of a factory that produces enzyme-washed blue jeans (the enzymes lighten the color of the denim, giving a faded appearance). When the most recent batch of fabric came out of the enzyme wash, however, the color wasn’t light enough to meet the standards. Your quality control laboratory wants to do some tests to determine why the wash enzymes didn’t perform as expected. Which hypothesis is most likely to be productive for the initial investigation?
The three-dimensional amino acid structure of the enzyme may have been altered
You’re the manager of a factory that produces enzyme-washed blue jeans (the enzymes lighten the color of the denim, giving a faded appearance). When the most recent batch of fabric came out of the enzyme wash, however, the color wasn’t light enough to meet the standards. Your quality control laboratory wants to do some tests to determine why the wash enzymes didn’t perform as expected. Based on your understanding of enzyme structure, which of the following would you recommend the lab also investigate?
The temperature of the liquid in the washing vat
The molecular formula for glucose is C6H12O6. What would be the molecular formula for a polymer made by joining 10 glucose molecules together by dehydration reactions?
C60H102O51
Which of the following pairs of base sequences could form a short stretch of a normal double helix of DNA?
5’-ATGC-3’ with 5’-GCAT-3’
Water is an excellent solvent. Select the property that justifies this statement.
As a polar molecule, it can surround and dissolve ionic and polar molecules.
If three molecules of a fatty acid (each with a formula of C6H22O2) are joined to a molecule of glycerol (C3H8O3), the resulting molecule would have the formula
C51H74O9
How do organisms manage to grow, reproduce, and maintain their internal organization?
Organisms exchange matter with the environment, taking in nutrients and releasing waste products
Solution A has a pH of 2 and solution B has a pH of 6. What is the difference between the number of H+ ions of these solutions?
Solution A has 10,000 more H+ ions than solution B.