Chapter 14- Chemical Kinetics
1/29
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
30 Terms
1st order overall, k =
time-1
2nd order overall, k =
M-1time-1
3rd order overall, k =
M-2time-1
units for rate
M/s or mol/L*s
How do you determine order of a reaction?
find the relationships between the concentration of the reactants and the rate (double concentration, double rate = 1 order); look at graph
As temperature/concentration/surface area/presence of a catalyst increases, the rate of the reaction/effective collisions
increase
As particle size increases, the rate of reaction/effective collisions
decreases
How does the reaction orders (rate law) for elementary steps relate to the Stoichiometry of the particles in that step?
they equal each other
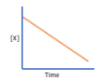
What order is this graph?
zero order reaction; [X] vs. time
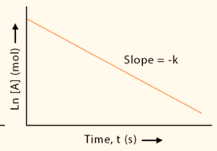
What order is this graph?
first order reaction; ln[X] vs. time
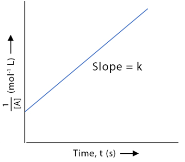
What order is this graph?
second order reaction; 1/[X] vs. time
How can a rate law explain the relationship between changes in reactant concentration and the rate of a reaction?
it shows the relationship in mathematical terms; k is constant so any increase in concentration, increases rate and vice versa; orders on the concentration shows the proportional relationship as if concentration doubles then rate quadruples in a second order reaction for that concentration
rate of reaction of appearance equation
(change in moles)/(change in time)
rate of reaction of disappearance equation
-(change in moles)/(change in time)
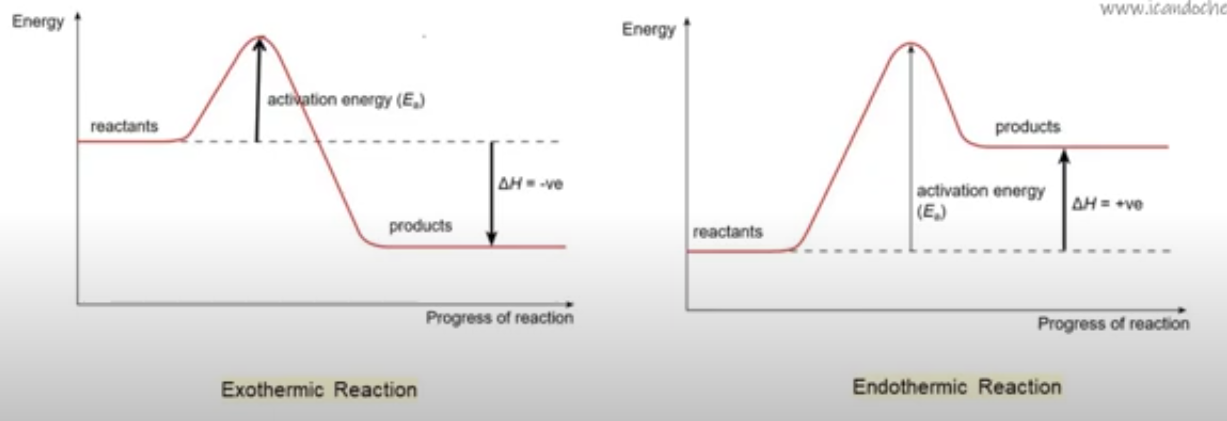
What is an energy profile?
shows the energy changes throughout a reaction
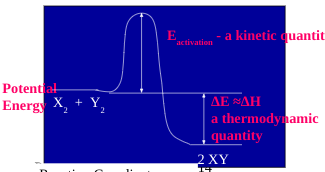
What features are in an energy profile?
activation energy, Ea (peak of the curve); reactant’s energy, products’ energy, and the difference between them (ΔH/ΔE); exothermic reactions have products lower in energy than reactants; endothermic reactions have higher energy products
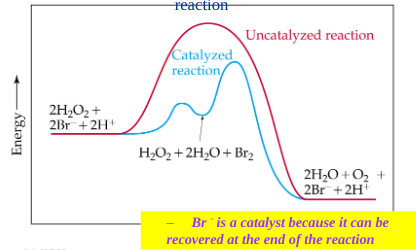
Hw do you identify a catalyst in a multistep mechanism?
appears as a reactant in an early step and reappears as a product in a later step without being consumed in the overall reaction
How do you identify an intermediate in a multistep mechanism?
species formed in one step and consumed in the next; don’t appear in overall balanced equation; ex: NO3(g)
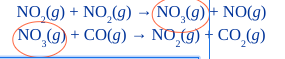
How do you determine rate law from a multistep mechanism?
use the slow step (that determines the reaction rate) by making a rate law from it; it will match the experimentally determined rate law of the overall reaction
What is a exothermic energy profile?
graph drops lower in energy from reactants to products
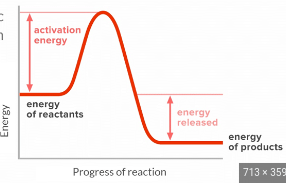
What is the endothermic energy profile?
graph rises higher in energy from reactants to products
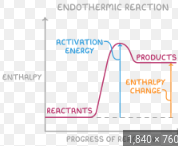
What is the forward reaction energy?
Ea; typically exothermic; lower the Ea, the faster the reaction
What is the reverse reaction energy?
Ea + ΔE; typically endothermic
half life, t1/2
time required for half of the reactant to be consumed
How can a reaction occur?
reactant molecules must collide in the correct orientation and with enough energy to form products
What are the two types of catalysts?
homogeneous and heterogeneous
What are homogeneous catalysts?
the catalyst and reactant are in the same phase
What are heterogeneous catalysts?
the catalyst and reactant are in different phases; ex: solid catalyst, gaseous reactants and products
Adsorption
binding of a reactant molecules to the catalyst surface; absorbed species are very reactive; molecules are absorbed onto active sites on the catalyst surface
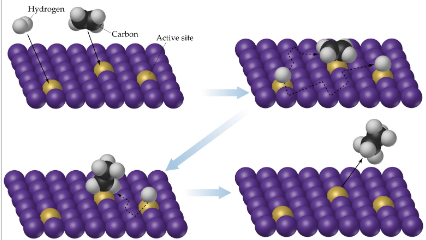
Catalyst
increase number of effective collisions; increase k by increasing A or decreasing Ea (Arrhenius equation); add intermediates to the reaction; very specific shapes and reactions; ex. enzyme- substrates undergo reaction at the active site of an enzyme (if it is open, locks into enzyme and a fast reaction occurs if not, catalyst is inhibited: enzyme inhibitors)