Lecture 5 - Colligative properties
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
Formula for describing how pressure and temperature change along coexistence curves
Clausius Clapeyron equation (is in formularium)
describes how the pressure of a substance changes with temperature during a phase change
Clausius Clapeyron equation for the boiling curve
Is also in formularium
Connects pressure and temperature on boiling curve.

Colligative properties
Properties that only depend on the number of molecules that are dissolved in a solution.
These properties are only valid when the concentration of solute is low enough to neglect interactions between molecules.
Examples of colligative properties
Vapor pressure lowering
Boiling point elevation
Freezing point depression
Osmotic pressure
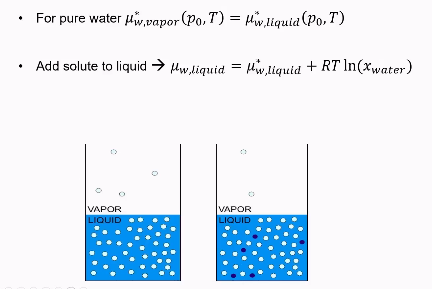
Vapor pressure lowering (example of colligative property)
as seen in the picture, blue particles are being added.
they are non-volatile, so they do no vaporize and stay in the liquid state.
Additionally, their concentration is low enough for it to be a colligative property.
The only thing happening is that the entropy is increasing.
As seen in the picture, before the blue particles were added. The chemical potential of vapor = potential of liquid.
The chemical potential of the mixed system is smaller than the chemical potential of the pure system due to entropy of mixing.
As a result, vapor particles will want to even out the imbalance and go into the liquid.
As a consequence, the number of molecules in the vapor phase will reduce. = lower pressure.
How to find the amount of reduction in pressure?
Using Raoult’s law.
Is in formularium.

Mole fraction for colligative properties in salts
Calculate moles for salt & water
Since salts split up into ions (e.g. two ions for NaCl) multiply the moles for NaCl by two.
Check picture
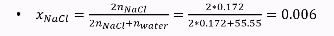
Henry’s law
The amount of gas that can be dissolved in a liquid
Important for carbonated drinks
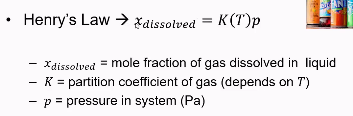
Principle of boiling point elevation (example colligative properties)
Adding salt to water → reduces chemical potential → increases boiling point
You can cook food faster as a result because you can use higher temperatures.
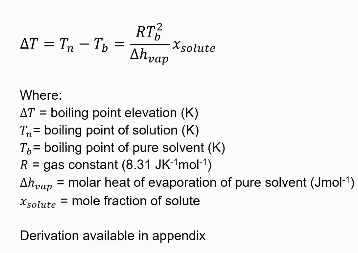
Boiling point elevation formula
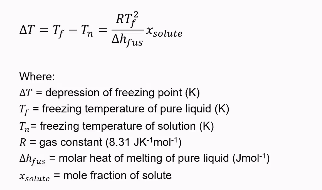
Principle of freezing point depression (example of colligative property)
When adding a solute the freezing point will decrease
Can be modelled by formula seen in picture
Freezing is governed by the chemical potential (μ) of the solvent:
At freezing, the chemical potential of the liquid solvent (μliquid) equals that of the solid solvent (μsolid).
Adding solute decreases μliquid (because solute increases entropy in the liquid phase).
To restore equilibrium (μliquid=μsolid), temperature must drop, further reducing μliquid.
Formula for calculating osmotic pressure
C solute = molar concentration
Remember that salt dissociates so n solute might be times two.
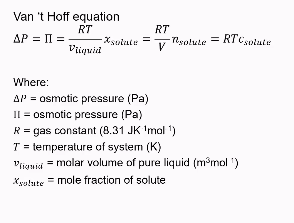
What effect does higher Mr have on osmotic pressure
The higher the Mr → the lower the amount of moles → the lower the change in atmospheric pressure.
however, some salts such as CaCl2 will split up into three ions. So you would add a factor of three to multiply the number of moles.
The larger the molecule, the lower the change in osmotic pressure.
Example osmosis:
Plant cells have osmotic pressure difference that gives structure.
If you put a cell wall in water, the cell walls will expand and eventually break.
If you put a cell wall in sugar, water will move out of the cell and the fruit will shrivel. Sugar molecules will diffuse into fruit.
Order of molar volume between solid, liquid, gas.
Gas = largest molar volume
liquid = second largest
Solid = smallest.
Difference between liquid and solid is small
then larger for gas & solid
largest for liquid & gas.
Is a lower or higher chemical potential better?
Just like Gibbs, lower.