Chemistry - Topic 2 - Bonding & Structure
1/116
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
117 Terms
what is ionic bonding?
the strong electrostatic attraction between oppositely charged ions or positive cations and negative anions
what is covalent bonding?
the electrostatic attraction between the positive nuclei of the bonded atoms and negative shared pair(s) of electrons
what is metallic bonding?
the electrostatic attraction between the positive metal ions and negative delocalised electrons
what is the octet rule and to which atoms does it apply to?
it states that each atom within a molecule will have a ‘full outer shell’ of electrons, taking into account both the shared pairs and the lone pairs.
it works for all molecules within which the central atom is in period 2 and for many molecules where the central atom is in period 3.
what does it mean to ‘expand its octet’ and for which atoms does this apply to?
if the central atom in a molecule is from period 3 (or below, so period 4, 5, etc), it can ‘expand its octet’, meaning it can have more than 8 valence electrons around it when covalently bonded with other atoms in the molecule.
the maximum number of covalent bonds that can be formed corresponds to the total number of electrons in the outer shell of the atom.
what is meant by the term isoelectronic?
same electronic configuration
what factors affect the radius of an ion?
nuclear charge
number of shells
shielding
what factors affect the strength of metallic bonding?
charge on the metal cation, bigger charge = stronger bonding
radius of the metal ion, smaller radius = stronger bonding
(number of delocalised electrons)
why does calcium oxide have a higer melting point than sodium fluoride?
both have giant ionic lattice structures
the ions present in CaO are Ca2+ and O2-, while the ions present in NaF are Na+ and F-
since the product of the charges of the metal cation and non-metal anion is of a greater magnitude for CaO than NaF (4 and 1 respectively), the electrostatic attraction between the cations and anions is stronger in CaO than in NaF
so more energy is required to overcome this
what is an ion?
an atom or a group of covalently bonded atoms that possesses an overall electric charge due to an imbalance of protons and electrons - this can occur via the gain or loss of electrons and H+ ions
what is a monatomic ion?
an ion formed from an individual atom (contains one atom)
what is a polyatomic ion?
an ion formed from two or more atoms covalently bonded together (contains multiple atoms)
how can we identify whether a compound is ionic based on its name and formula?
metal + non-metal
if it contains nitrate, sulphate, carbonate, hydroxide, ammonium (known to be polyatomic ions)
which is larger, an oxide ion or a sulphide ion?
sulphide ion (S2-) is larger than oxide ion (O2-)
greater ionic radius
more shells
greater shielding effect
(despite increased nuclear charge)
outer electron less attracted to nucleus
why are the ions close-packed?
this corresponds to maximum net attraction between all the ions in the lattice
describe the structure of an ionic compound
giant ionic lattice
regular, repeating three-dimensional arrangement (lattice) of alternating cations and anions (continuous)
this pattern extends indefinitely (giant - continuous structure) throughout the structure
how are ionic compounds held together?
by ionic bonding, which is the strong electrostatic attraction between oppositely charged ions
how are polyatomic ions formed from molecules?
via the transfer of H+ ions (protons)
dative covalent bond has formed
heterolytic bond fission
explain whether chlorine or bromine has a bigger atomic radius
Br
it has more shells
hence more shielding (more core electrons)
despite having a higher nuclear charge
the outer electrons feel a lower net electrostatic attraction to the nucleus
hence they are further away
which is larger, a chloride ion or a sulphide ion?
Cl- and S2- are isoelectronic (same electronic configuration)
same number of shells
similar shielding effect
chlorine has a greater nuclear charge
outer electron more attracted to nucleus in Cl-
so Cl- has a smaller radius
what is required for two atoms to covalently bond with one another?
non-metals
the overlap of singly-occupied atomic orbitals
why are atoms of the noble gas elements unable to covalent bond with one another?
they have no singly-occupied orbitals (they are all full)
which factors will affect the strength of a covalent bond?
number of shared pairs of electrons
distance between the shared electrons and the nuclei (atomic radii of the bonded atom)
suggest why the O = O bond is stronger than the F - F bond.
the O = O bond has 2 shared pair of electrons whereas F - F has only 1 shared pair of electrons
so there is stronger electrostatic attraction between the nuclei of the bonded atoms and shared pair of electrons
therefore the O = O bond is shorter and stronger
hence more energy is required to overcome this
suggest why the Cl - Cl bond is stronger than the Br - Br bond.
chlorine has a smaller atomic radius
shorter distance between nuclei and shared electrons in Cl - Cl (shorter bond length)
so stronger electrostatic attraction (more energy required to overcome)
why does nitrogen have a lower boiling point than oxygen, despite the N ≡ N bond being much stronger than the O = O bond?
covalent bonds are not broken in this situation
oxygen (O2)
why is salty water a better conductor of electricity than pure water?
salty water contains Na+ ions and Cl- ions
these are charged particles which are free to move
the concentration of ions in salty water is much higher than in pure water (which only contains a very small concentration of H+ and OH- ions)
which atoms strictly follow the octet rule?
H, C, N, O and F
i.e. (period 1 and period 2 non-metals except for B and the noble gases)
which type of bonding is present in silicon dioxide + name the structure of silicon dioxide?
covalent
giant covalent structure
which type of bonding is present in carbon dioxide + name the structure of carbon dioxide?
covalent
simple molecular structure
what is dative covalent bonding?
a covalent bond (shared pair) where both electrons come from one of the atoms involved
what is meant by the word molecule?
two or more atoms covalently bonded together
neutral particle
any molecule can be assigned a molecular formula
what is the formula for calculating the formal charge of atoms in a molecule?
- FC = V - N - B/2
V is the number of valence electrons in the neutral atom
N is the number of non-bonded valence electrons associated with the atom
B is the number of bonded (shared) valence electrons associated with the atom
what key factors will affect the strength of electrostatic attraction between the ions?
the magnitude of the charge of each ion (the product of the charges) - greater charges = stronger attraction
the sum of the ionic radii - bigger radii = weaker attraction
why do both NaF and MgO have very high melting points?
both of these compounds have a giant ionic lattice structure
in which there is strong electrostatic attraction between the oppositely charged ions (many strong ionic bonds)
this attraction must be overcome when these compounds melt, and a large amount of energy is required to do this
why is the melting point of potassium iodide lower than that of sodium fluoride?
potassium iodide contains K+ and I- ions, whereas sodium fluoride contains Na+ and F- ions
K+ ions are larger than Na+ ions because they have more shells, and I- ions are larger than F- ions for the same reason.
the larger ionic radii of the ions in KI means there is weaker electrostatic attraction between them
this means less energy is required to overcome this attraction (in order for KI to melt)
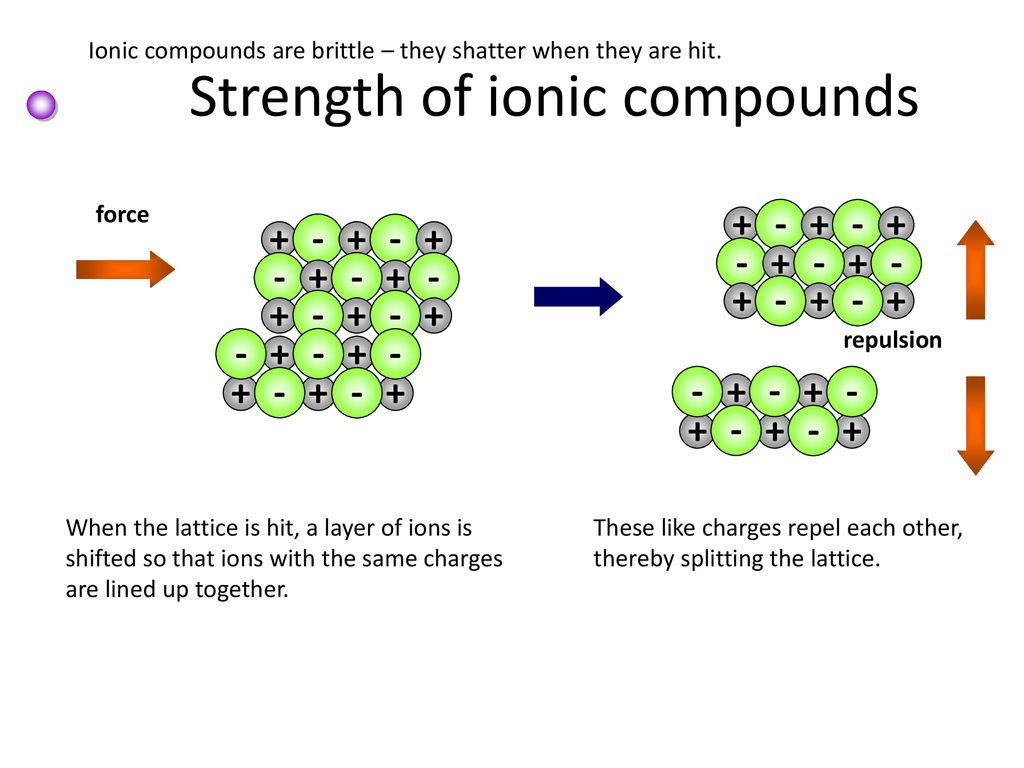
why are ionic compounds brittle? draw a diagram to demonstrate this behaviour.
when a force is applied, ions of the same charge are moved such that they align with one another
this means they repel each other
(which causes the structure to fall apart)
what is electronegativity?
the ability of an atom to attract electron density/the shared bonding pair of electrons towards itself in a covalent bond
what type of bonding is present in iodine and what type of structure does iodine have?
covalent bonding
simple molecular structure
evidence for the existence of ions - what colour are maganate and potassium ions + what is the slide soaked in and why?
MnO₄⁻ - purple
K⁺ - colourless
slide is soaked in KNO₃ solution which means the ions are aqueous and thus free to move
what colour is copper (II) chromate + the ions inside this compound
green compound
Cu²⁺ - blue when dissolved in water
CrO₄²⁻ - yellow when dissolved in water
how do the physical properties of ionic compounds provide evidence for the existence of ions?
high melting and boiling point
his could be caused by the strong electrostatic attraction between oppositely charged ions
electrical conductivity when solid or liquid
this can be explained if the individual ions are not free to move when solid, but become free to move when liquid
brittleness
this can be explained if the structure is composed of alternating positive and negative ions. applying a force could disrupt this arrangement and cause the structure to fall apart
solubility
(will come back to)
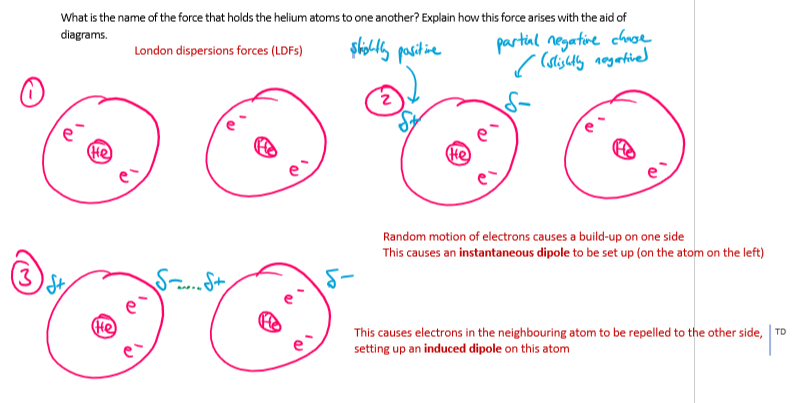
helium has a simple monatomic structure - what is the name of the force that holds the helium atoms to one another? explain how this force arises with the aid of diagrams.
London dispersion forces
random motion of electrons causes a build-up on one side of the atom
this causes an instantaneous dipole to be set up
this causes electrons in the neighbouring atom to be repelled to the other side , setting up an induced dipole
suggest an explanation for the observed increase in melting and boiling points as the group is descended
as the group is descended,
there are more electrons per atom
this means the London dispersion forces (LDFs) between them are stronger (as there are greater partial +ve and -ve charges on each atom, leading to stronger instantaneous and induced dipoles)
so more energy is required to overcome these LDFs
explain why pentane, methylbutane and dimethylpropane have similar boiling points
they are all alkanes, their molecules are non-polar
this means the molecules are held together by London dispersion forces (LDFs)
the number of electrons per molecule is the same in each case (5x6 + 12 = 42)
so the LDFs are similar in strength and require a similar amount of energy to overcome
explain the trend in boiling points observed - branching
as the amount of branching increases, boiling point decreases
this is because more branching means there are fewer points of contact between the molecules
which means the London forces are weaker
so less energy is required to overcome them
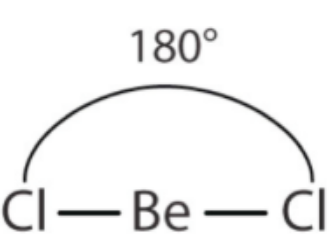
draw a diagram of the 3-D shape of BeCl₂ (beryllium chloride) + name the molecular shape + state the bond angle(s)
linear (around the Be atom)
2 bonding pairs and 0 lone pairs of electrons
180 degrees
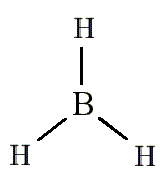
draw a diagram of the 3-D shape of BH₃ (borane) + name the molecular shape + state the bond angle(s)
trigonal planar (around the B atom)
3 bonding pairs and 0 lone pairs of electrons
120 degrees
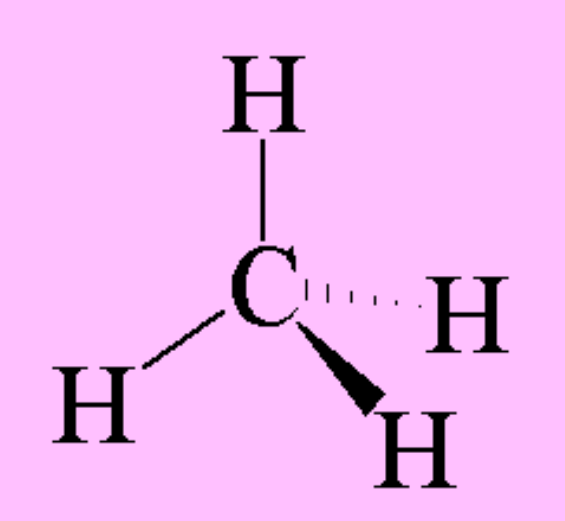
draw a diagram of the 3-D shape of CH₄ (methane) + name the molecular shape + state the bond angle(s)
tetrahedral (around the carbon atom)
4 bonding pairs and 0 lone pairs of electrons
109.5 degrees
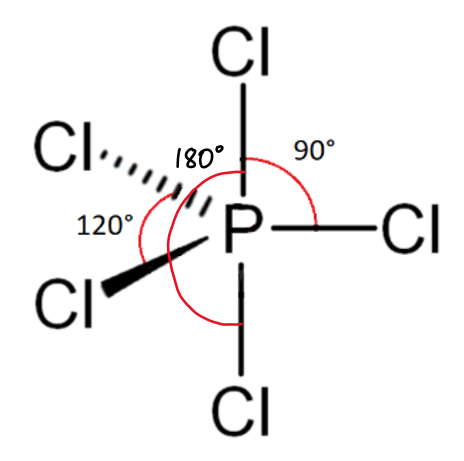
draw a diagram of the 3-D shape of PCl₅(g) (phosphorus pentachloride) + name the molecular shape + state the bond angle(s)
trigonal bipyramidal
5 bonding pairs and 0 lone pairs of electrons
90 degrees
180 degrees
120 degrees
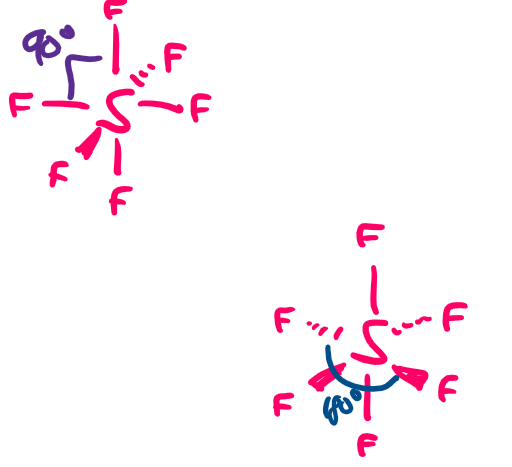
draw a diagram of the 3-D shape of SF₆ (sulphur hexafluoride) + name the molecular shape + state the bond angle(s)
octahedral
6 bonding pairs of electrons and 0 lone pairs of electrons
90 degrees
180 degrees
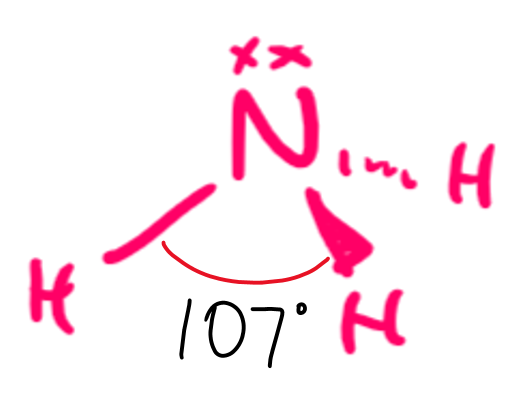
draw a diagram of the 3-D shape of NH₃ + name the molecular shape + state the bond angle(s)
trigonal pyramidal
3 bonding pairs of electrons and 1 lone pair of electrons
107 degrees
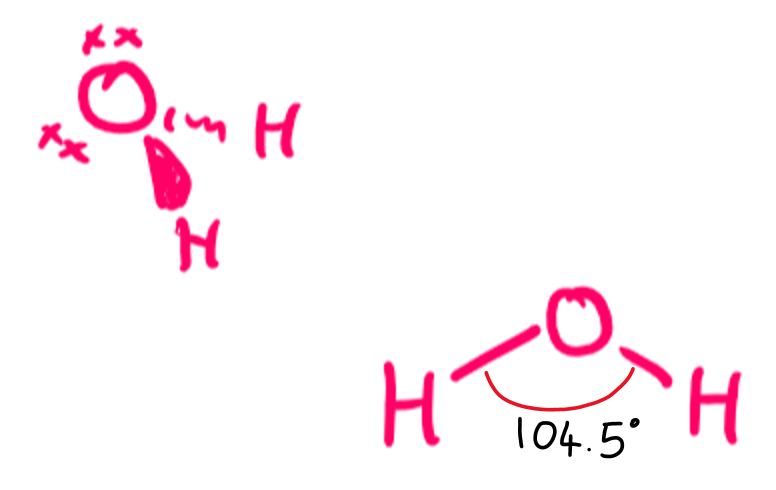
draw a diagram of the 3-D shape of H₂O + name the molecular shape + state the bond angle(s)
V-shaped or bent
2 bonding pairs and 2 lone pairs of electrons
104.5 degrees
what is the name for a covalent bond that involves two of the same atoms?
a homonuclear covalent bond
what is the name for a covalent bond that involves two different atoms?
a heteronuclear covalent bond
what makes a molecule polar?
the existence of a permanent dipole in the molecule
as one atom in the molecule has a stronger attraction for the shared pair of electrons than the other (more electronegative)
when can molecules contain polar bonds but still be non-polar overall?
when they are symmetrical
this occurs because the individual bond dipoles (vectors) cancel each other out if the molecule is symmetrical
what are allotropes?
different structural modifications of an element (namely carbon)
describe the structure of diamond
giant covalent structure
each atom covalently bonded to all of its neighbours, all atoms in the structure are connected by a network of covalent bonds
in diamond, each carbon atom is (singly) bonded to four others in a tetrahedral arrangement
explain why diamond is a poor conductor of electricity
diamond does not have any delocalised electrons or ions that are free to move through its structure
all valence electrons are localised in sigma bonds between the atoms
explain why graphene is extremely strong
graphene has a giant covalent structure
to break graphene, strong covalent bonds must be broken
these bonds require a large force to break, meaning graphene is strong
what type of force would be present between the layers in graphite?
London Dispersion Forces
why is diamond hard whereas graphite is soft/slippery?
diamond is hard because it has a rigid, 3D structure in which each carbon atom is bonded to four others
covalent bonds must be broken to break diamond and this requires a large amount of force/energy
graphite is soft because it has a layered structure (within each 2D layer, each carbon atom is bonded to three others)
the graphene layers are held to one another by comparatively weak LDFs
this means a comparatively small amount of force is required to cause to layers to slide over each other (meaning graphite is soft)
by considering its properties, suggest four uses for graphite
electrodes (because it is a good electrical conductor)
solid lubricant (because it is soft and slippery)
crucible (because it can withstand very high temperatures without melting/subliming)
pencils (because layers of graphene slide off easily)
explain why C60 fullerene sublimes at much lower temperatures than diamond or graphite
C60 has a simple molecular structure whereas diamond and graphite have giant covalent structures
subliming C60 requires the London dispersion forces between the C60 molecules to be overcome whereas, for diamond and graphite, covalent bonds must be broken
LDFs are much weaker than covalent bonds so less energy required to overcome them
why does C60 fullerene sublime at significantly higher temperatures than the boiling point of iodine
C60 molecule contains 360 electrons, whereas I2 molecule contains 106 electrons
so the London Dispersion Forces in C60 are stronger, more energy required
explain why C60 fullerene is a poor conductor of electricity
no delocalised electrons free to move throughout the simple molecular structure (through all the molecules)
no ions (hence no freely moving ions)
molecules are neutral
what is the structure of silicon + why does silicon have the highest melting point in period 3?
giant covalent structure
silicon has a giant covalent structure (similar to diamond) in which every atom is connected to four others through single covalent bonds which are very strong. When silicon melts, these covalent bonds must be broken and a very large amount of energy is required to do this
explain why the boiling point of silicon is extremely high?
silicon has a giant covalent structure
(it has the same structure as diamond - each silicon atom is singly bonded to four others in a tetrahedral arrangement)
there are many strong covalent bonds between the Si atoms in the structure that must be broken in order for silicon to sublime
this requires lots of energy
in which type of molecules are permanent dipole-permanent dipoles (pd-pd) forces present?
polar molecules
this is an additional intermolecular force present as well as the London forces between these molecules
why does ethane (CH3CH3) have a higher boiling point than fluorine (F2)?
Fluorine:
Lowest boiling point
Non-polar
LDFs only
18 electrons per F2 molecule
Ethane:
Non-polar
LDFs only
18 electrons per ethane molecule
Ethane molecules are larger than F2 molecules (contain more atoms) which means there is a greater surface area of contact with neighbouring molecules
So stronger LDFs than in fluorine (more energy to overcome)
when talking about LDFs remember to mention electrons…
per molecule!!
what conditions are required for hydrogen bonding to be present between two molecules? draw a diagram showing the hydrogen bonding present in water.
a hydrogen atom covalently bonded to a highly electronegative atom (fluorine, oxygen or nitrogen)
a lone pair of electrons on a second electronegative atom (N, O or F)
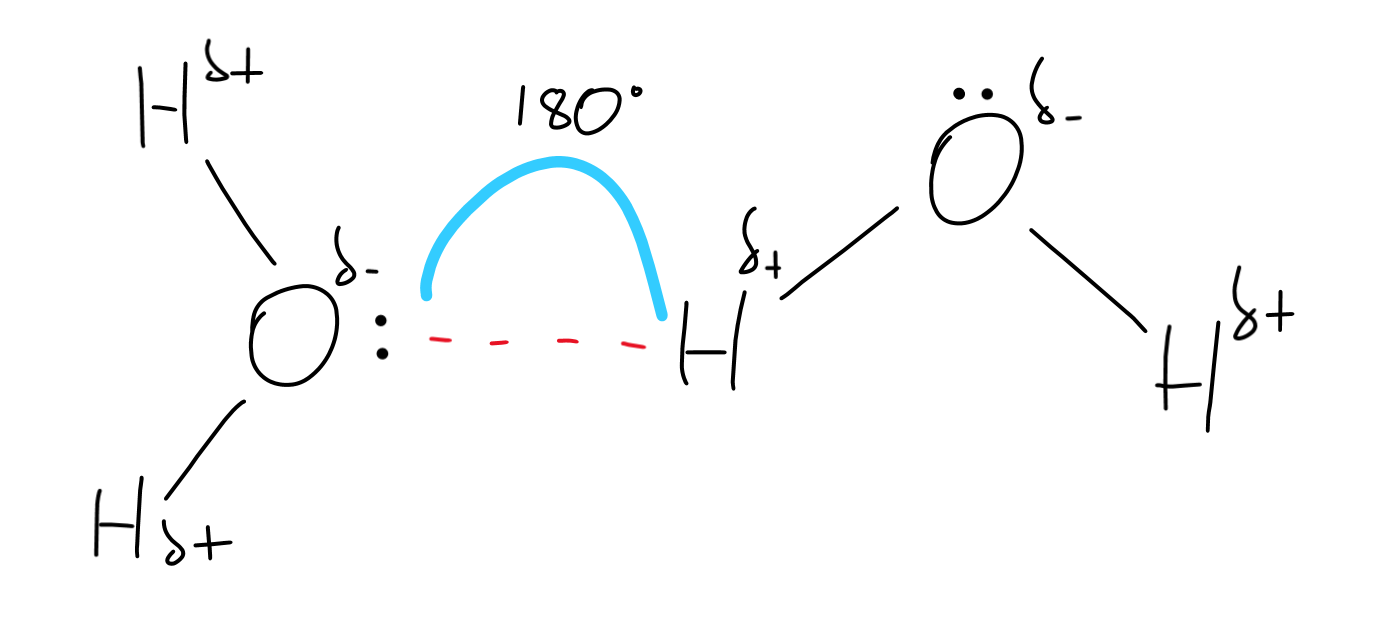
explain why hydrogen fluoride has the highest boiling point of the hydrogen halides
the intermolecular forces between the molecules is strongest in HF
because hydrogen bonding, which is an extremely strong type of i.m.f. exists only in HF but not in the other hydrogen halides (which counteracts the fact that the LDFs are weakest in HF)
(HF also has the strongest pd-pd forces)
therefore more energy required to overcome i.m.f.s in HF than in other hydrogen halides
why is ethanol miscible with water?
hydrogen bonds in water and ethanol must be overcome
hydrogen bonds are formed between water molecules and ethanol molecules (once mixed)
the forces formed are similar in strength to the forces overcome
so the energy released (when hydrogen bonds are formed between ethanol and water molecules) sufficiently compensates for the energy absorbed (when hydrogen bonds are overcome in water and ethanol)
why is cyclohexane immiscible with water?
hydrogen bonds in water and London forces in cyclohexane must be overcome
London forces are the strongest force that can be formed between water molecules and cyclohexane molecules (once mixed)
the forces formed are significantly weaker than the forces overcome
so the energy released forming forces does not sufficiently compensate for the energy absorbed overcoming forces
why is bromine soluble in hexane?
London forces in bromine and London forces in hexane must be overcome
London forces are formed between bromine molecules and hexane molecules (once mixed)
the forces formed are similar in strength to the forces overcome
so the energy released forming forces sufficiently compensates for the energy absorbed overcoming forces
why does sodium chloride have a high solubility in water?
electrostatic attraction between Na⁺ and Cl⁻ ions in NaCl and hydrogen bonds in water must be overcome
the Na⁺ and Cl⁻ ions are hydrated by water molecules once dissolved
the forces formed are similar in strength to the forces overcome
so the energy released forming forces sufficiently compensates for the energy absorbed overcoming forces
why does sodium chloride have a very low solubility in cyclohexane?
cyclohexane molecules are unable to hydrate the ions
because cyclohexane molecules are non-polar
the forces formed are significantly weaker than the forces overcome
so the energy released forming forces does not sufficiently compensate for the energy absorbed overcoming forces
predict the bond angle in chloric (I) acid, explain your answer (5)
104.5 degrees
2 bond pairs and 2 lone pairs (of electrons in valence shell of the oxygen atom)
valence electron pairs at minimum repulsion
lone pair repulsion>bond pair repulsion
so tetrahedral bond angle reduced/109.5 degrees angle reduced
describe how you might carry out an experiment to test whether a liquid is polar. (3)
stream of hydrogen peroxide liquid
idea of charging a rod
put near ‘stream’ and stream is deflected if polar
the bond angles in hydrogen peroxide are similar to those in a water molecule. suggest a bond angle for hydrogen peroxide and reasons for your value. (3)
104.5 degrees
electron pairs repel to maximum separation/minimum repulsion
lone pairs repel more than bonded pairs
deduce the value of the S-C-S bond angle in CS₂. justify your answer. (3)
180 degrees
2 sets of bonding electrons/regions of (bonding) electron density
which repel to maximum separation/minimum repulsion
state and explain whether the electronegativity of fluorine is greater than, similar to or less than, that of bromine. hence explain why hydrogen fluoride can form hydrogen bonds but hydrogen bromide cannot. (3)
fluorine atom is more electronegative
because it has less shielding/(bonding) electrons closer to the nucleus
so HF has a (greater) dipole moment
explain why the HNH bond angle in NH₃ is less than that for FBF in BF3
3 electron pairs around central B atom but 4 electron pairs around central N atom (hence less space)/ammonia has an extra pair of electrons around N
explain why the bond angle in water is less than the bond angle in ammonia.
oxygen has one more lone pair of electrons than nitrogen
so the repulsion from the oxygen lone pairs is greater (and reduces the bond angle)
use the two different features of ammonia and boron trifluoride to explain how a dative covalent bond is formed.
donation of lone pair of electrons from nitrogen
to the boron atom which is electron deficient/has only six electrons in its outer shell/can accept two electrons to complete its octet
why does boron trifluoride have a trigonal planar shape with bond angles of 120 degrees?
3 bonding pairs of electrons
(the bonding pairs of electrons) move apart to minimise repulsion
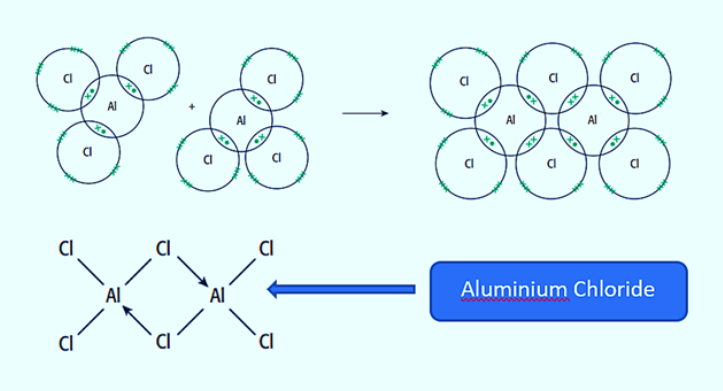
draw a dot-and-cross diagram for a molecule of Al₂Cl₆, showing outer electrons only. what type of bond is formed?
two dative covalent bonds are formed
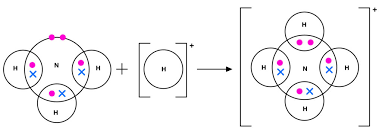
use dot-and-cross diagrams to illustrate how an ammonium ion is made from ammonia, showing outer electrons only. what type of bond is formed?
a dative covalent bond is formed.
draw a diagram of the 3-D shape of NH₄⁺ + name the molecular shape + state the bond angle(s)
tetrahedral (around the nitrogen atom)
4 bonding pairs and 0 lone pairs of electrons
109.5 degrees

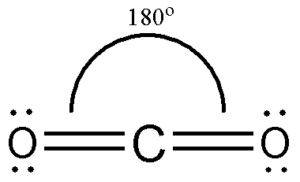
draw a diagram of the 3-D shape of CO₂ + name the molecular shape + state the bond angle(s)
linear (about the C atom)
4 bonding pairs and 0 lone pairs of electrons BUT 2 regions of bonding electrons
180 degrees
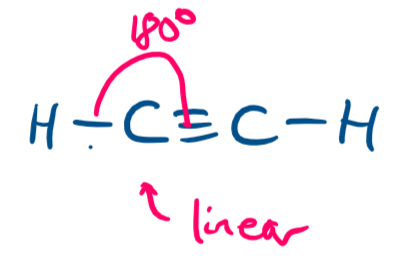
draw a diagram of the 3-D shape of C₂H₂ (ethyne) + name the molecular shape about each C atom + state the bond angle(s)
linear (about each C atom)
in outer shell of each C atom there are 4 bonding pairs and 0 lone pairs of electrons BUT more importantly 2 regions of bonding electrons
180 degrees
what type of bonding does H₂O contain?
London forces
permanent dipole-dipole forces
hydrogen bonding
what type of bonding occurs between NH₃ molecules?
London forces
permanent dipole-dipole forces
hydrogen bonding
why does water have an unusually high melting and boiling point compared to similarly sized molecules?
water contains hydrogen bonding due to the presence of the -OH group
hydrogen bonding is a very strong type of intermolecular force
therefore more energy is required to overcome the hydrogen bonding in water in order for it to melt/boil
why do alcohols have a relatively low volatility (how easily a substance will vaporise) compared to alkanes with a similar number of electrons?
low volatility - higher boiling temperatures
because alcohols contain hydrogen bonding while alkanes do not
why does ice have a much lower density than liquid water?
hydrogen bonds in ice hold molecules in a rigid structure with lots of air gaps, as hydrogen bonds push molecules further apart, which lowers the density
each water molecule forms 4 hydrogen bonds with adjacent molecules
hydrogen bond lengths are relatively long
molecules are arranged in an open/hexagonal lattice structure
held further apart than in water
what type of substance is ice, H₂O, and what type of structure does ice have?
ice is a covalently bonded substance
with a simple molecular structure
explain why the bonds within the hydrogen sulphide molecule are polar. (1)
the atoms (that the bond is between) have differing electronegativity values