Angiogenesis, Invasion and Metastasis
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
What is angiogenesis?
Formation of new blood vessels from pre-existing vasculature.
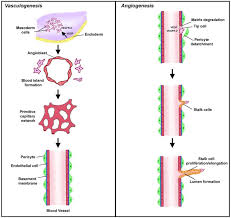
What is vasculogenesis?
Formation of new blood vessels from stem cell differentiation.
Only in specialised cancers (e.g. brain cancer)
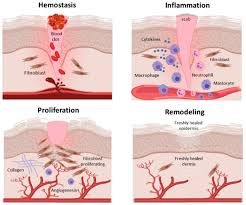
There are similarities in angiogenesis between cancer and ___________.
wound healing
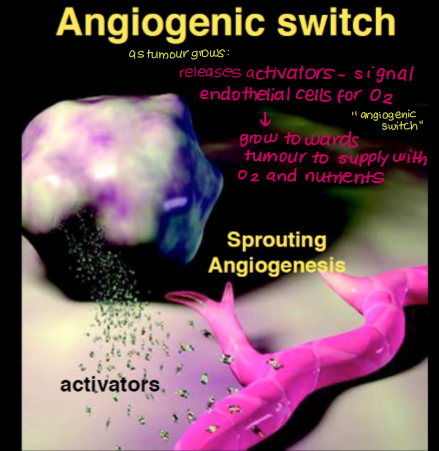
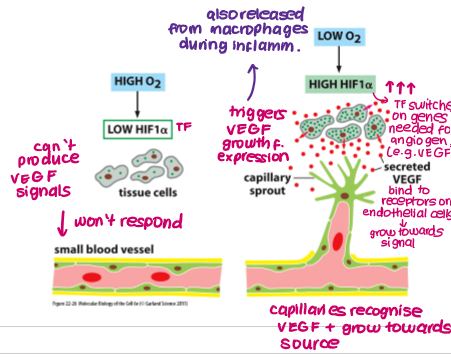
Describe the angiogenic switch.
Hypoxia/lack of nutrients stimulates increased expression of HIF1a (TF) in tissue/cancer cells
HIF1a induces expression of genes required for angiogenesis (e.g. VEGF)
VEGF binds to VEGF receptors (RTK) on endothelial cells in pre-existing vessels
Capillaries grow towards source of signalling molecules
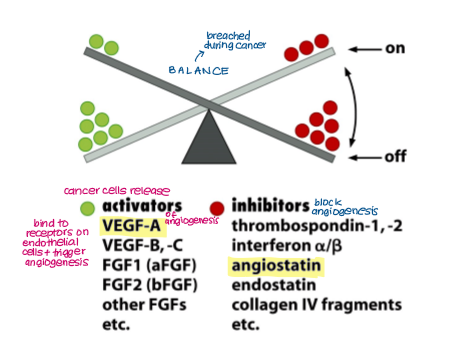
Angiogenesis is maintained by a balance between activators and inhibitors, which is breached during cancer. Give examples.
Activators:
VEGF A, B, C
FGF1 and 2
Inhibitors:
Angiostatin
Thrombospondin
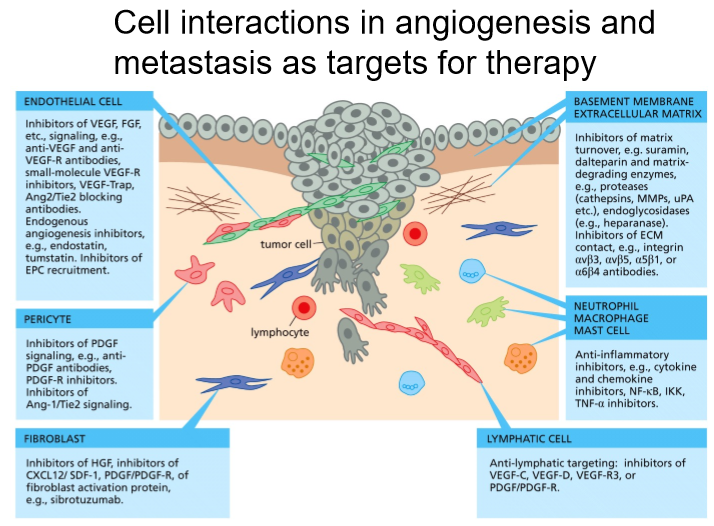
Angiogenesis involves different types of cells:
Cancer cells: secrete VEGF
CSCs: may differentiate into endothelial cells in some cancers
Inflammatory cells: activate VEGF and allow contact with endothelial cells
Endothelial cells: contain VEGF receptors, proliferate in response
Pericytes: contractile cells that surround endothelial cells, cause vessel leakiness (more oxygen, nutrients leak out, promote cancer growth)
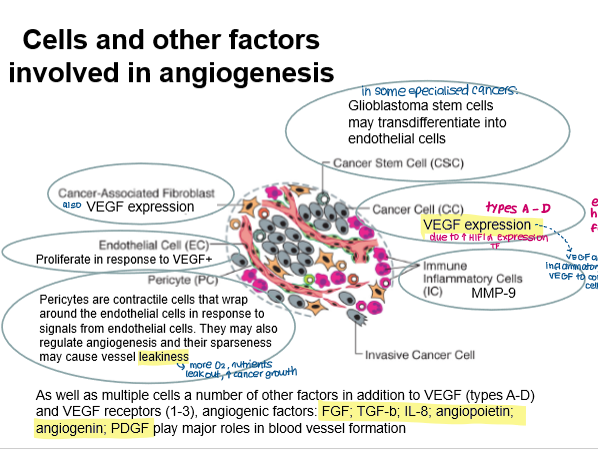
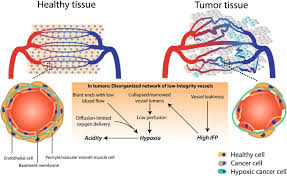
How do leaky vessels arise and how does this affect cancer cell growth?
Pericytes surround endothelial can be sparsely distributed
Leaky vessels increase oxygen and nutrient supply for cancer cells.
How many types of VEGF are there?
4
VEGF A, B, C, D
How many types of VEGF receptors are there?
3
Monoclonal antibody treatments targeting angiogenesis (e.g. Avastin) have been developed. However, these are not effective. Why?
many factors involved in angiogenesis (e.g. inflammatory cells, pericytes, endothelial cells, many types of VEGF and receptors)
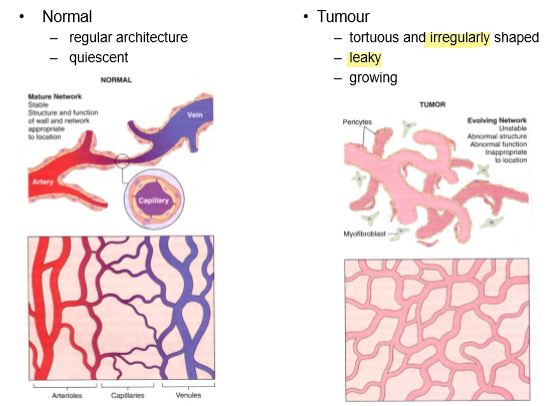
What are the main differences between angiogenesis in normal tissue and in cancer?
Tumour vessels are irregular, numerous and leaky.
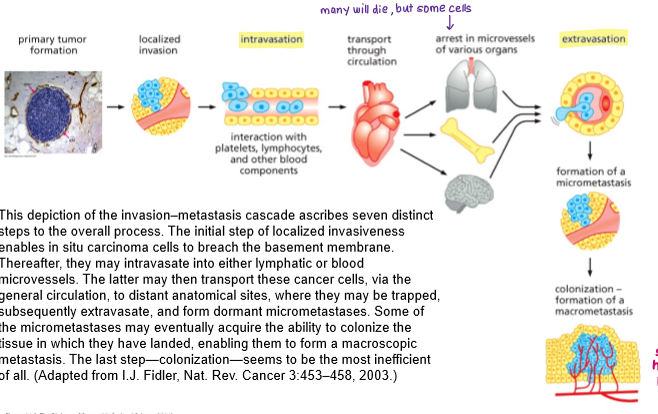
Summarise the invasion-metastasis cascade.
1. Local invasion: Loss of basement membrane- Cancer cells lose normal cell–cell adhesion (e.g. reduced E-cadherin), undergo cytoskeletal changes and degrade the basement membrane and extracellular matrix using proteases such as matrix metalloproteinases. This allows cells to invade surrounding tissue.
2. Intravasation: Invasive tumour cells enter nearby blood or lymphatic vessels. They interact with platelets and other blood components to survive.
3.Cells are transported through circulation. Many die, but some arrest (get trapped) in microvessels of various organs.
4. Extravasation: Cancer cells adhere to the endothelium at distant sites and exit the circulation by crossing the vessel wall into surrounding tissue.
5. Colonisation and outgrowth: Disseminated tumour cells adapt to the new microenvironment, survive as dormant cells or micrometastases (single cancer cells), and eventually proliferate to form clinically detectable macrometastases.
Local invasion of cancer cells requires degradation of _______ and _________. This happens via __________.
basement membrane and extracellular matrix (ECM)
fibroblasts and stromal cells degrade the ECM
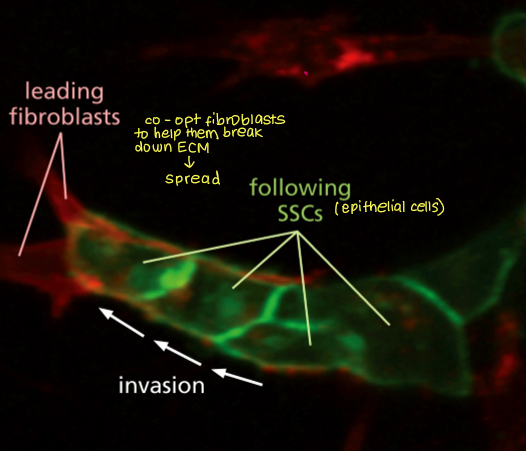
Intravasation can be influenced by which other cells? How does this happen?
macrophages
remodel the ECM through secretion of proteases, creating paths for cancer cells to reach blood vessels. They also increase vascular permeability by releasing factors such as VEGF and TNF-α, making it easier for tumour cells to cross the endothelial barrier.
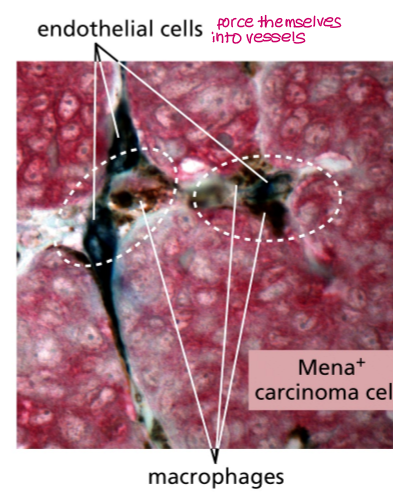
In the blood vessels, circulating tumour cells interact with?
platelets
They rapidly coat tumour cells, forming platelet–tumour aggregates that shield CTCs from immune recognition and stress.
Platelet-derived adhesion molecules help CTCs attach to the endothelium, facilitating arrest and subsequent extravasation at distant sites.
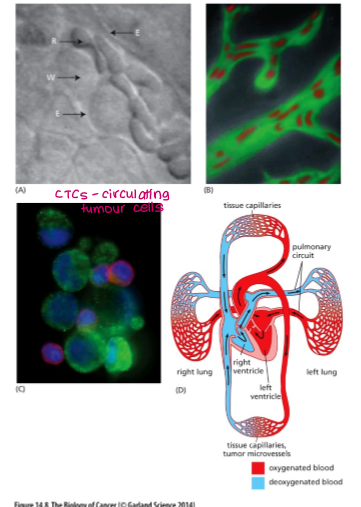
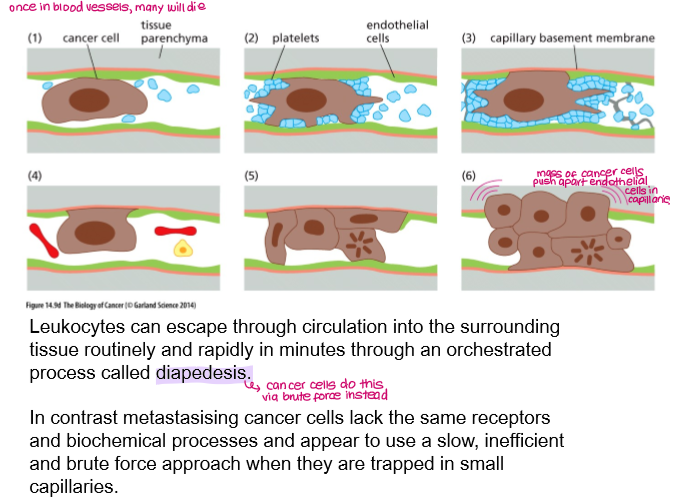
How do cancer cells extravasate the blood vessels?
brute force
Arrest & Adhesion:
CTCs get trapped in narrow capillaries, often due to size.
They form weak, then stable, attachments with the endothelial cells lining the vessel wall via platelets.
Trans-Endothelial Migration (TEM):
They then penetrate the endothelial barrier.
ECM Invasion:
Once past the endothelium, cells interact with and degrade the extracellular matrix (ECM) using enzymes to create a path into the new tissue.
Colonization:
Extravasated cells either remain dormant or proliferate to form new metastatic tumors, influenced by the secondary site's microenvironment.
Invasion and metastasis is due to ____________ rather than genetic mutations.
changes in gene expression (EMT/MET)
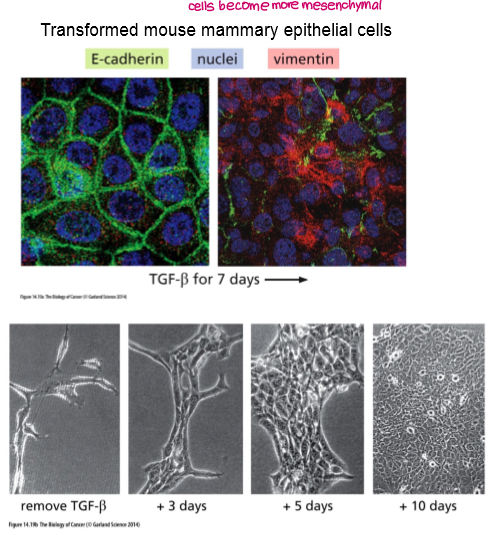
What is EMT?
Epithelial Mesenchymal Transition
Epithelial cells acquire phenotypes of mesenchymal cells such as fibroblasts.
Normal part of development but co-opted by cancer cells to facilitate invasion and metastasis.
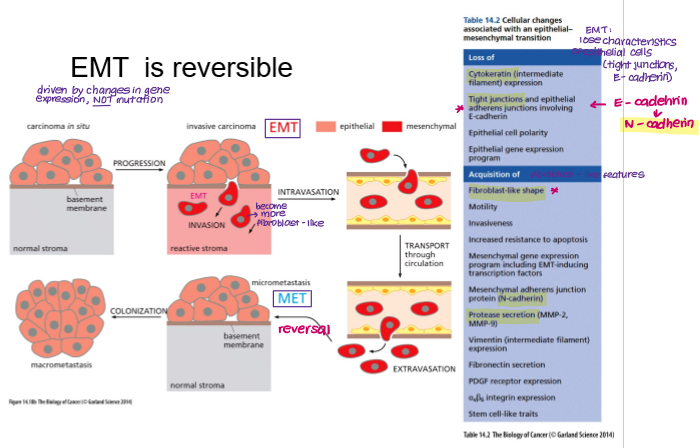
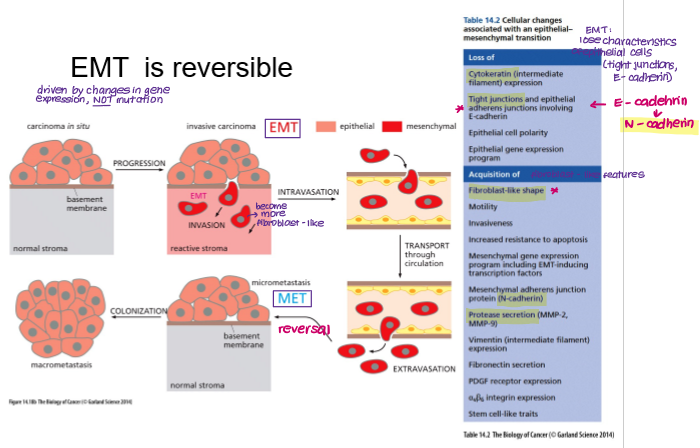
EMT is reversible. What is this process called?
MET
Mesenchymal Epithelial Transition
What are the key cellular changes associated with EMT?
Loss of E-cadherin → gain of N-cadherin
Loss of tight junctions
Gain of metalloproteases (e.g. MMP-2)
Gain of fibroblast-like shape and motility
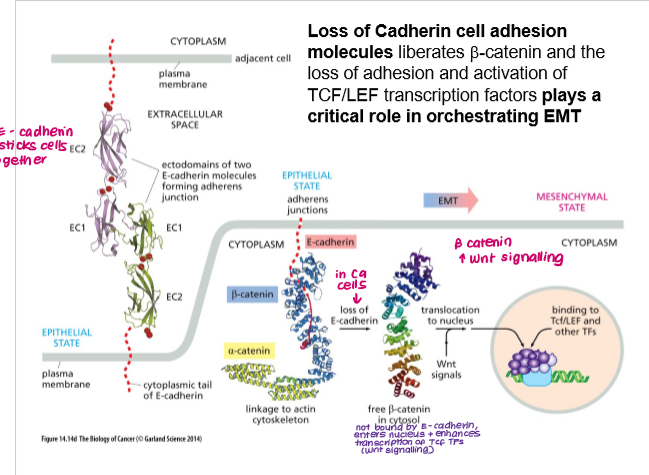
How does loss of E-cadherin contribute to EMT and invasion?
Loss of E-cadherin:
beta-catenin no longer bound to membrane
beta-catenin migrates to nucleus and induces expression of TFs (Tcf) → EMT
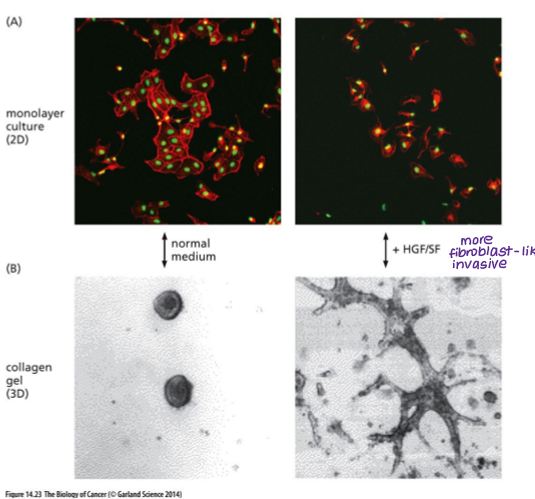
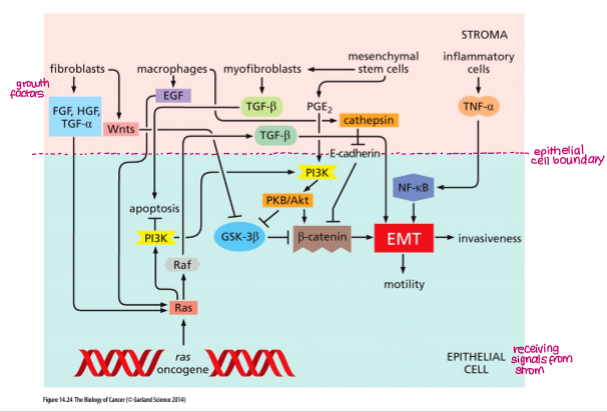
Another growth factor that can induce invasive behaviour is?
HGF (hepatocyte growth factor)
Induces EMT
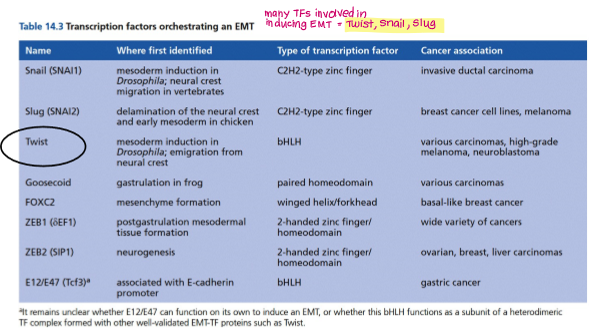
Give examples of TFs that can induce EMT and invasion.
Twist
Snail
Slug
Which pro-inflammatory cytokine also induces EMT and invasion?
TGF-b
In summary, EMT can be induced by a range of transcription factors, growth factors and other molecules, including:
Loss of E-cadherin (activation of TCF)
TFs (Twist, Snail, Slug)
HGF (hepatocellular growth factor)
TGF-b
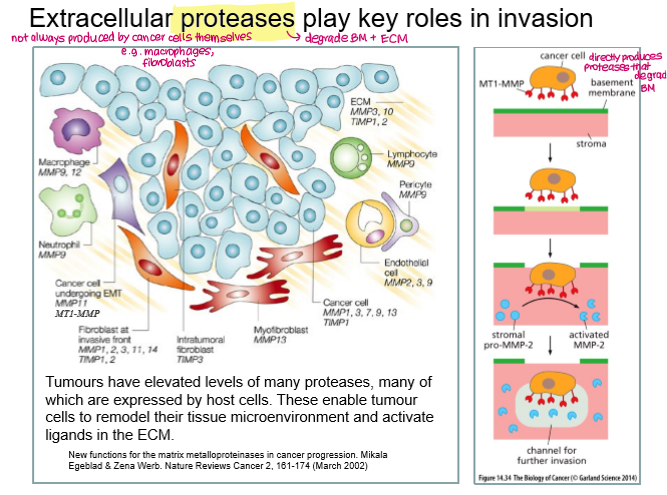
__________ play a key role in invasion by degrading the extracellular matrix.
Extracellular proteases- can be produced by cancer cells themselves or other cells (e.g. macrophages, fibroblasts)
e.g. MMP-1 (degrade collagens), MMP-2
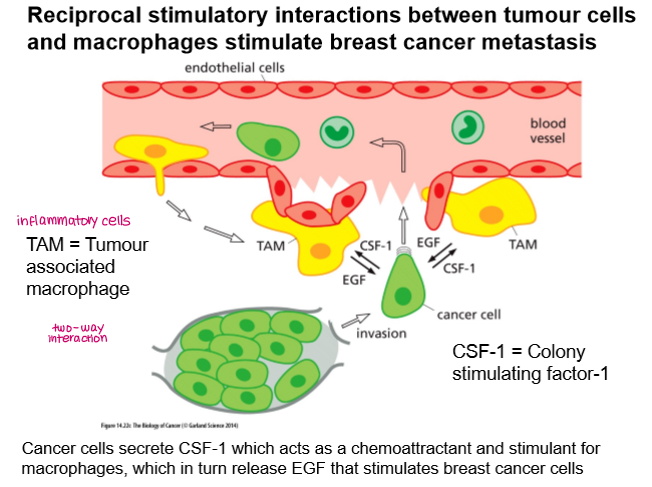
Metastasis of breast cancer is stimulated by:
two-way interaction between tumour cells and macrophages (Tumour-Associated Macrophages)
Tumour cells secrete CSF-1 which acts as a chemoattractant for macrophages, which in turn release EGF that stimulates breast cancer cells.
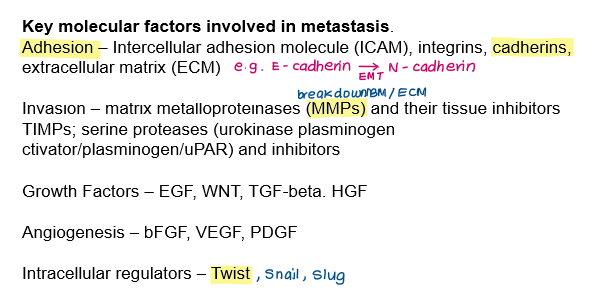
Key molecular factors involved in metastasis
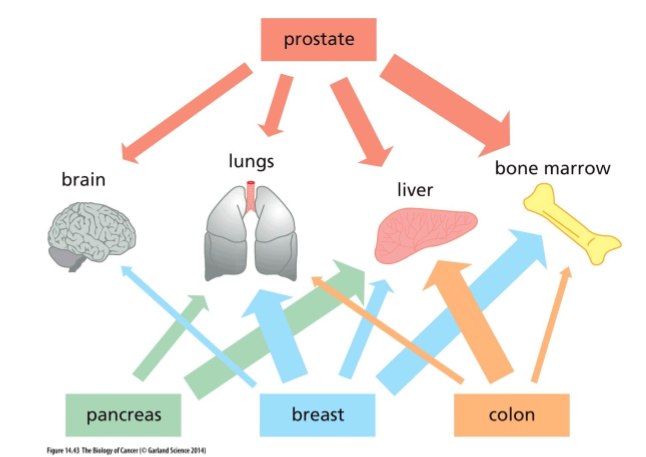
Primary colon cancers typically metastasise into which organ?
liver
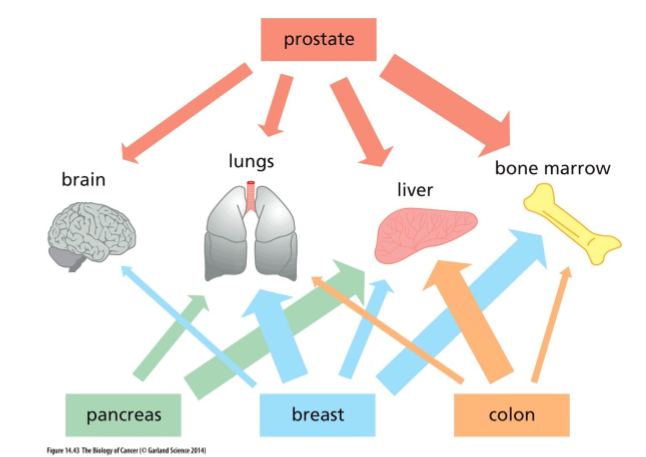
Primary pancreatic cancers typically metastasise into which organ?
liver
Cell interactions in angiogenesis and metastasis as targets for therapy.