Ch 3: Biological Molecules
1/71
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
72 Terms
Monomers
The individual, small units that link together to form larger molecules.
Polymers
Large molecules (macromolecules) composed of many repeating monomer units.
Examples of Polymers
Include polysaccharides, polypeptides (proteins), DNA, and RNA.
Macromolecules
Very large molecules, generally defined as having a molecular weight greater than 1000 daltons.
Hydrophobic
"Water-fearing": Describes non-polar molecules or parts of molecules that tend to be excluded from aqueous solutions.
Hydrophilic
"Water-loving": Describes polar or charged molecules or parts of molecules that are soluble in water.
Condensation Synthesis (Dehydration reaction)
The process by which monomers combine to form dimers and higher-order polymers, involving the removal of an -H from one monomer and an -OH from another. They create bonds between monomers in carbohydrates, lipids, and proteins.
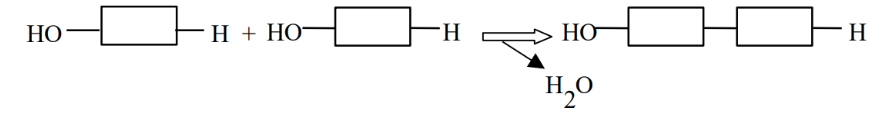
Hydrolysis
The process of breaking down dimers, trimers, or polymers back into monomers by adding an -H and an -OH group derived from the splitting of a water molecule. Hydrolysis is vital for catabolic processes that release energy stored in macromolecules, and it is performed by specific enzymes

Carbohydrates
Simple sugars made of Carbon, Hydrogen, and Oxygen in a ratio of C:H2:O, primarily produced by photosynthetic organisms.
Monosaccharides
The monomer units of carbohydrates, with carbon skeleton sizes ranging from 3 to 7 carbons.
Aldose Sugar- Monosaccharide
Has the carbonyl group (-C=O) at the end of the molecule (e.g., glucose).
Ketose Sugar- Monosaccharide
Has the carbonyl group (-C=O) in the middle of the molecule (e.g., fructose).
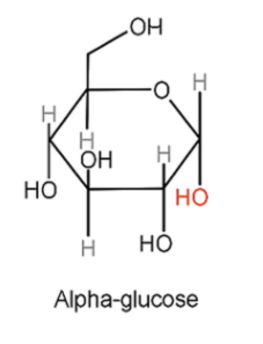
α-glucose (alpha-glucose)
The -OH group produced on carbon 1 is positioned below the plane of the ring.
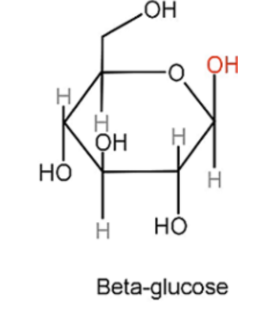
β-glucose
The -OH group produced on carbon 1 is positioned above the plane of the ring.
Functions of Monosaccharides
They are a major source of energy for cells (e.g., glucose and fructose provide about 4 kcal/g).
Energy stored in their bonds is harvested through glycolysis and respiration.
Their carbon skeletons are also used to synthesize other molecules.
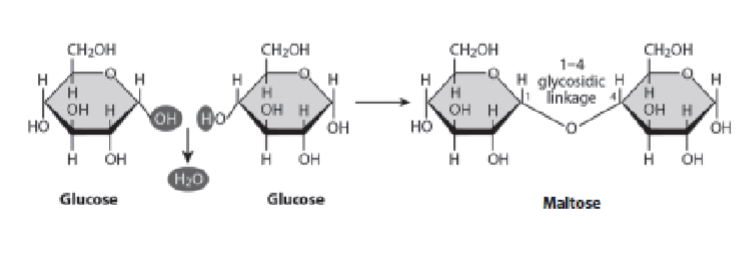
Disaccharides
Formed by the enzymatic combination of two monosaccharides through glycosidic linkages. A glycosidic linkage involves a carbon on the first sugar, an oxygen in the middle, and a carbon on the second sugar.
Examples of Disaccharides
Include maltose (glucose + glucose)
lactose (glucose + galactose)
sucrose (glucose + fructose).
α-1,4 glycosidic linkage (Disaccharide)
Formed when two α-glucose molecules combine (as in maltose).
β-1,4 glycosidic linkages
Formed when β-glucose molecules combine (as in cellulose).
Oligosaccharides
Shorter chains of carbohydrates, typically composed of about 5 to 20 sugar molecules. They can be covalently linked to proteins or lipids and may function as regulatory molecules.
Polysaccharides
Long carbohydrate chains, containing up to several thousands of monomer units.
Storage Polysaccharides
Used for energy utilization.
Starch (Storage Polysaccharides)
α-1,4 linked glucose, found in plants as amylose and amylopectin; a major storage product in crops like potato, rice, wheat, and corn.
Glycogen (Storage Polysaccharides)
Similar to starch but highly branched, also α-1,4 linked glucose; stored in the liver and muscles of animals.
Structural Polysaccharides
Used to build cell walls and exoskeletons.
Cellulose Structural Polysaccharides
Composed of β-1,4 linked glucose molecules; the primary constituent of plant cell walls and the most abundant biopolymer on Earth, possessing tremendous mechanical strength.
Callose (Structural Polysaccharides)
Shorter than cellulose, contains β-1,3 linked glucose molecules; formed at wounding sites in plants.
Chitin (Structural Polysaccharides)
Consists of β-1,4 linked N-acetyl glucosamine; a major structural component of the exoskeletons of insects and the cell walls of fungi.
Glucose Ring Structure
A glucose ring structure with proper numbering of carbons, where Carbon 1 is to the right of the ring oxygen, and subsequent carbons are numbered sequentially.
Lipids
A diverse class of biological molecules that are not classified as polymers and are generally non-polar.
Fatty Acids (Categories of lipids)
Components of many lipids, their hydrocarbon chain structure determines properties.
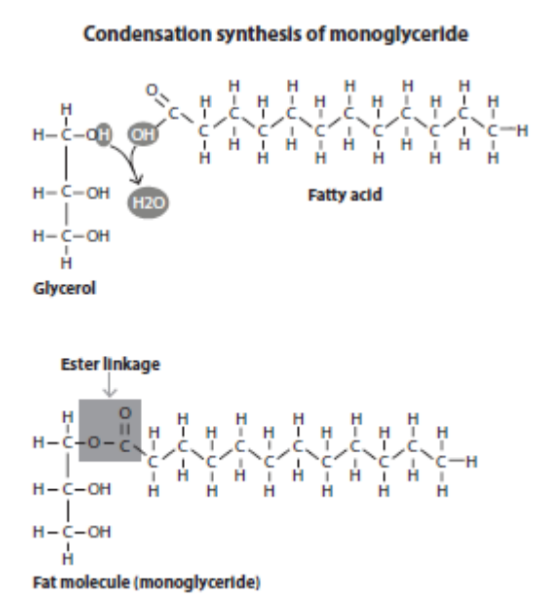
Triglycerides (Fats) (Categories of lipids)
Formed from glycerol and three fatty acids; their primary function is energy storage.
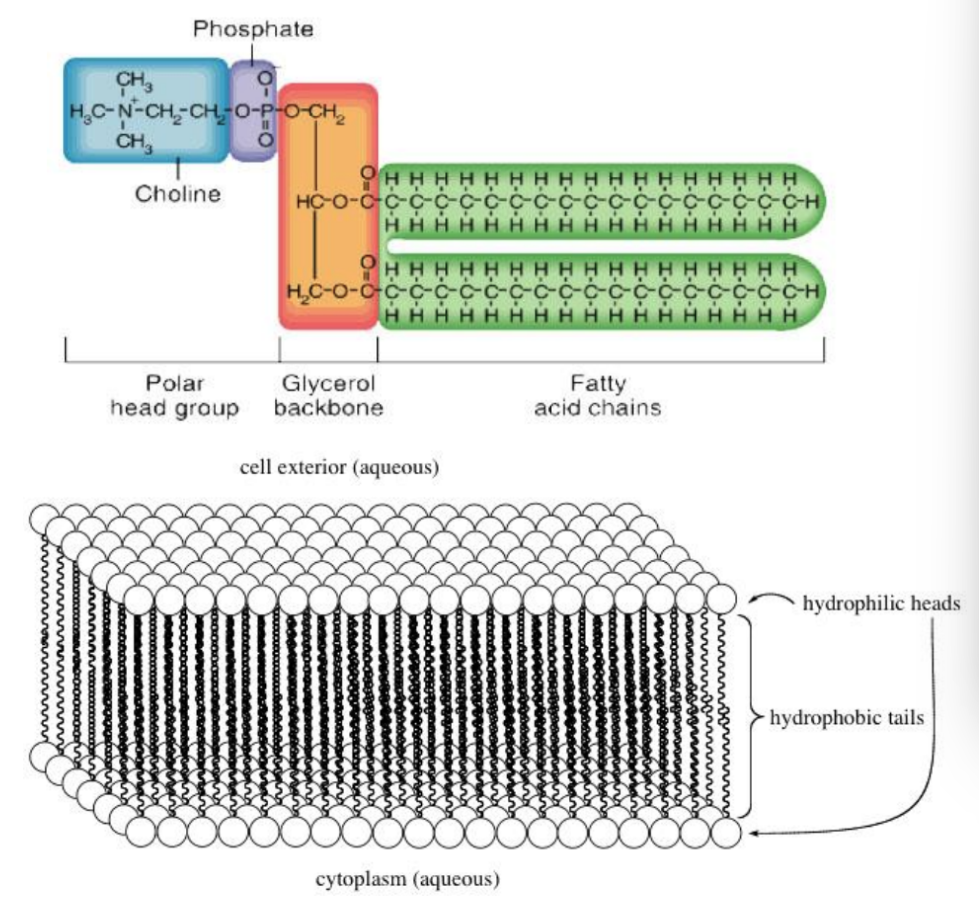
Phospholipids
Amphipathic molecules (have both hydrophilic and hydrophobic parts) that form the main structural components of biological membranes.
Functions of Lipids
Include energy storage, structural components of membranes, and signaling.
Saturated Fats
Have no double bonds between carbons in their hydrocarbon chains.
Unsaturated Fats
Contain one or more double bonds between carbons in their hydrocarbon chains; the presence of these double bonds can cause 'kinks' in the chain, and make it more fluid. These fats can be found in plant oils and are typically liquid at room temperature. .
Triglyceride Formation
The structures of one glycerol molecule and three fatty acids reacting to form a triglyceride, showing the ester linkages that connect the fatty acids to the glycerol.
Phospholipid Structure
Identifies its different parts: a polar head group containing a phosphate, and two hydrophobic hydrocarbon tails.
Membrane Bilayer Assembly
Properties of phospholipids lead to the self-assembly of a membrane bilayer, with hydrophilic regions facing the aqueous environment and hydrophobic regions concealed within the bilayer.
Proteins
Macromolecules made of amino acid monomers.
Functions of Proteins
Diverse roles including acting as enzymes (catalysts) for biochemical reactions, providing structural support, facilitating transport, and serving regulatory and signaling roles.
3-D Shape of Proteins
The overall 3-D shape of a protein is critical for its optimal function.
Basic Structure of an Amino Acid
An amino acid consists of a central carbon atom bonded to an amino group (-NH2), a carboxyl group (-COOH), a hydrogen atom (-H), and a variable 'R' group (side chain).
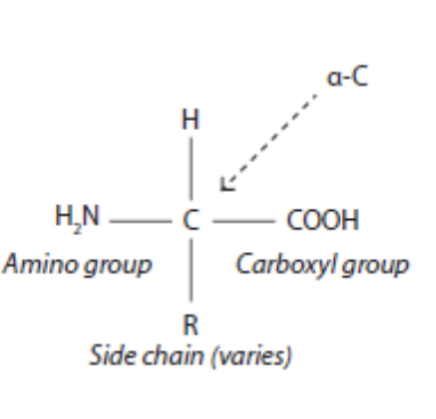
Amino and Carboxyl Groups
It is acceptable for the amino and carboxyl groups to be drawn as either charged (-NH3+, -COO-) or uncharged forms.
Properties of Amino Acids
The general features of the side chain (R group) determine the amino acid's properties (e.g., polar, non-polar, charged).
Dipeptide Formation
Two amino acids react to form a dipeptide, showing the formation of the peptide bond (a covalent bond) that connects the two amino acid residues.
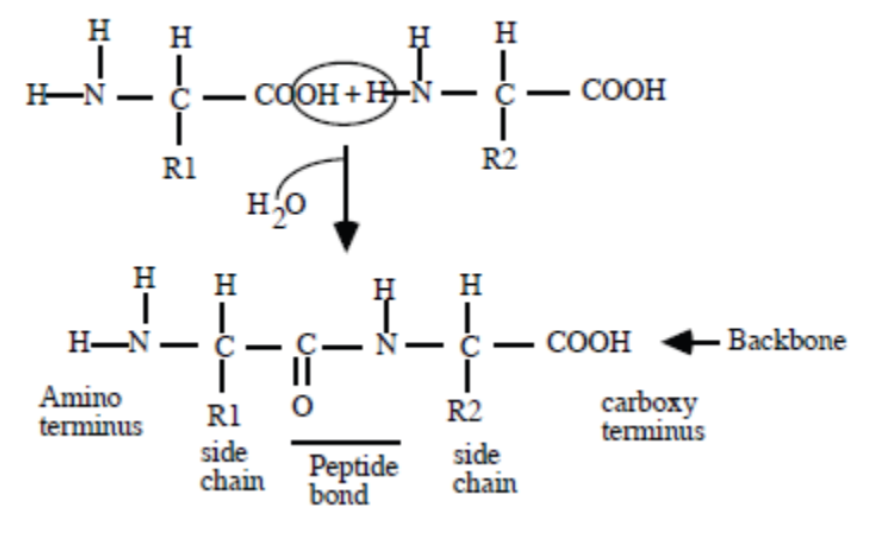
N-terminal and C-terminal Ends
The N-terminal end is the end with the free amino group, and the C-terminal end is the end with the free carboxyl group of the dipeptide.
Denaturation
Causes a protein to unfold and become nonfunctional.
Primary Structure of Proteins
The unique linear sequence of amino acids in a polypeptide chain, held together by peptide bonds (covalent bonds).
Secondary Structure of Proteins
Localized, regular folding patterns within the polypeptide chain, such as alpha-helices and beta-sheets, stabilized by hydrogen bonds formed between atoms of the polypeptide backbone.
Tertiary Structure of Proteins
The overall, three-dimensional shape of a single polypeptide chain, stabilized by various interactions between the R groups.
Quaternary Structure of Proteins
The three-dimensional arrangement of multiple polypeptide chains (subunits) to form a functional protein complex.
Polypeptide Backbone
The repeating sequence of atoms (N-C-C) that forms the core chain of a polypeptide, excluding the R groups.
Disulfide Bond (-S-S-)
A strong covalent bond formed between the sulfhydryl (-SH) groups of two cysteine amino acid residues, crucial for stabilizing the tertiary (and sometimes quaternary) structure of proteins.
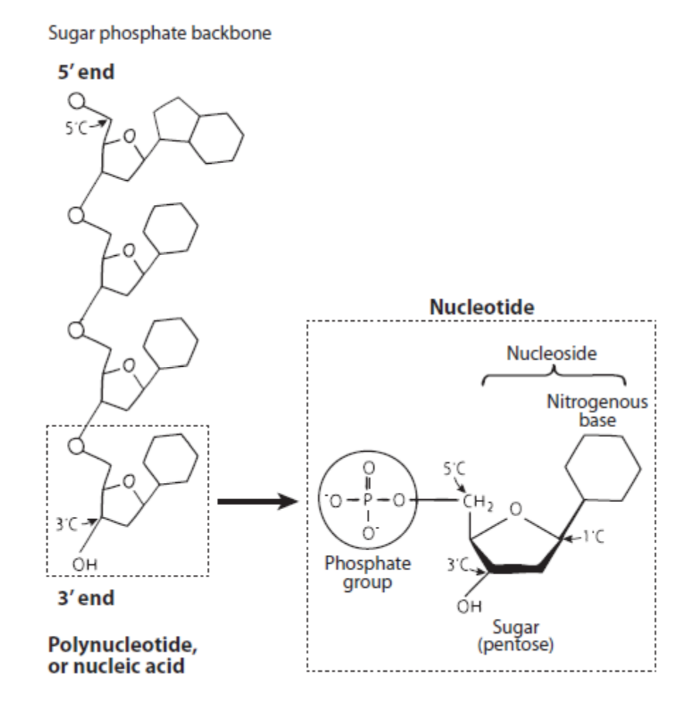
Nucleic Acids
Polymers made of nucleotide monomers.
DNA Composition
DNA (Deoxyribonucleic Acid) is made of monomers called deoxyribonucleotides, which contain a nitrogenous base, a deoxyribose sugar (lacks an -OH group at the 2' carbon), and a phosphate group.
Nitrogenous bases in DNA
The nitrogenous bases in DNA are Adenine (A), Guanine (G), Cytosine (C), Thymine (T)
RNA Composition
RNA (Ribonucleic Acid) is made of monomers called ribonucleotides, which contain a nitrogenous base, a ribose sugar (has an -OH group at the 2' carbon), and a phosphate group.
Nitrogenous Bases in RNA
The nitrogenous bases in RNA are Adenine (A), Guanine (G), Cytosine (C), and Uracil (U). Uracil replaces Thymine in RNA.
DNA Structure
DNA primarily exists as a double-stranded double helix with two strands running in antiparallel orientation (one 5' to 3', the other 3' to 5')
Base Pairing in DNA
Base pairing occurs via hydrogen bonds: Adenine (A) pairs with Thymine (T), and Guanine (G) pairs with Cytosine (C).
Stability of DNA
DNA is more stable than RNA due to its double-stranded nature and the absence of the 2'-OH group.
RNA
Mostly exists as single-stranded molecules, but it can form complex and variable secondary structures (regions where it folds back on itself and forms double-stranded sections).
Base pairing is complementary
Adenine (A) pairs with Uracil (U), and Guanine (G) pairs with Cytosine (C) via hydrogen bonds.
DNA
Its primary function is to store genetic information in its sequence and code for RNA. It is replicated and passed on from generation to generation.
Functional Differences of RNA
Copies information from DNA, and these RNA copies either function directly in the cell (e.g., ribosomal RNA, transfer RNA) or are used to direct the synthesis of proteins. Some RNA molecules (ribozymes) can even have catalytic activity.
Nucleoside
A nitrogenous base linked to a ribose sugar.
Nucleotide
A nitrogenous base linked to a ribose sugar and one or more phosphate groups (e.g., NMP, NDP, NTP).
NTPs/dNTPs
NTPs (nucleotide triphosphates) and dNTPs (deoxyribonucleotide triphosphates), with three phosphates, are the active monomers used for RNA and DNA synthesis, respectively.
ATP
Adenosine triphosphate is a key nucleotide that serves as the major form of cellular energy.
Drawing NTP and dNTP Monomers
Be able to draw a generic NTP (for RNA synthesis) and a generic dNTP (for DNA synthesis). For the nitrogenous base, you can simply write 'Base' or 'Nitrogenous Base' instead of drawing its specific structure.
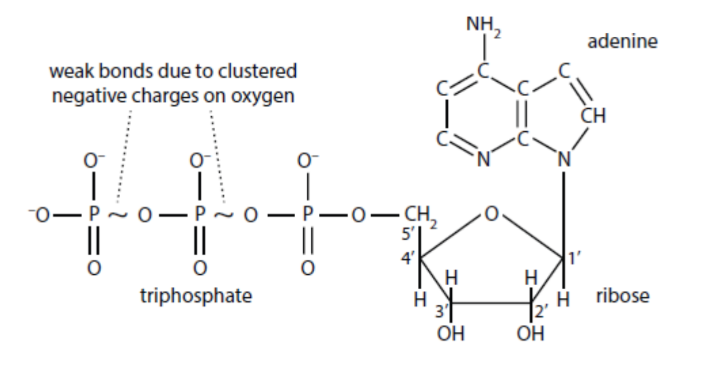
Polynucleotide Strand
Be able to draw a polynucleotide strand showing two nucleotides joined together. Indicate the numbering on the sugar rings (1' to 5'). Clearly show whether there is an -OH or an -H group at the 2' position on the ribose sugar, as this distinguishes RNA (2'-OH) from DNA (2'-H). Show the phosphodiester linkage (or phosphodiester bond) that connects the 5' carbon of one nucleotide to the 3' carbon of the next. Mark the 5' end (with a free 5' carbon group) and the 3' end (with a free 3' carbon group) of the strand.