ch 19 practice Q's TCA CYCLE
1/33
Earn XP
Description and Tags
BIOCHEM UNIT 3
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
The TCA cycle occurs in which cellular compartment?
A. Cytosol
B. Mitochondrial matrix
C. Mitochondrial intermembrane space
D. Nucleus
B. mitochondrial matrix
the TCA cycle enzymes are located in the mitochondrial matric, where they have access to acetyl-CoA and can efficiently couple NADH-FADH2 production to the electron transport chain in the inner membrane
how many NADH molecules are produced per acetyl-CoA in one complete turn of the TCA cycle
A. 2
B. 3
C. 4
D. 6
B. 3
three NADH molecules are generated per turn: one at isocitrate dehydrogenase, one at α-ketoglutarate dehydrogenase, and one at malate dehydrogenase.
which enzyme catalyzes the first step of the TCA cycle?
A. Aconitase
B. Isocitrate dehydrogenase
C. Citrate synthase
D. Succinate dehydrogenase
C. Citrate synthase
citrate synthase catalyzes the condensation of acetyl-CoA with oxaloacetate to form citrate, initiating the cycle.
why is the TCA cycle called an “amphibolic pathway?
A. it occurs in both prokaryotes and eukaryotes
B. It serves both catabolic and anabolic functions
C. It can run in both directions
D. It produces both NADH and FADH₂
B. it serves both catabolic and anabolic functions
the TCA cycle is amphibolic because it both breaks down acetyl-CoA for energy (catabolic) and provides intermediates for biosynthesis of amino acids, heme, and other molecules (anabolic).
what is the primary function of anaplerotic reactions?
A. To remove excess intermediates from the cycle
B. To replenish TCA cycle intermediates that have been removed for biosynthesis
C. To speed up the TCA cycle
D. To convert NADH back to NAD⁺
B. to replenish TCA cycle intermediates that have been removed for biosynthesis
anaplerotic reactions (like pyruvate carboxylase converting pyruvate to oxaloacetate) replenish cycle intermediates that have been siphoned off for biosynthetic pathways, maintaining cycle function.
high levels of ATP would have what effect on TCA cycle activity?
A. Increase cycle activity
B. Decrease cycle activity
C. No effect on cycle activity
D. Reverse the direction of the cycle
B. decrease cycle activity
high ATP signals that the cell has sufficient energy, so the TCA cycle slows down through negative feedback regulation of key enzymes like isocitrate dehydrogenase and α-ketoglutarate dehydrogenase.
true or false: the TCA cycle directly produces more ATP/GTP molecules than NADH molecules per turn
false
the cycle produces only 1 GTP/ATP (at succinyl-COA synthetase) but produces 3 NADH molecules per turn, making NADH the major direct product
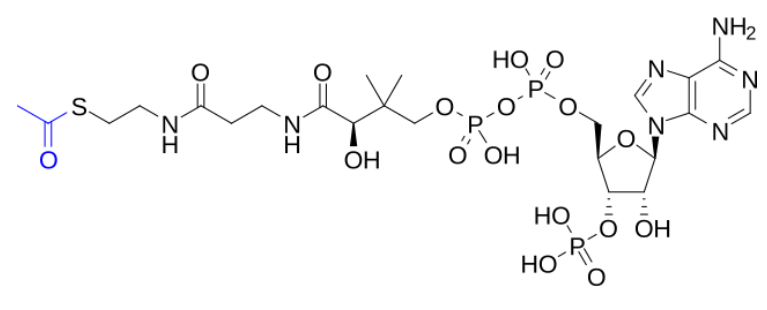
true or false: Acetyl-CoA can cross the mitochondrial membrane directly. the protonated structure of acetyl-CoA is shown
false
acetyl-CoA cannot cross membranes directly due to its charged CoA group. this is why citrate is exported to the cytosol for fatty acids synthesis, where it’s cleaved to regenerate acetyl-CoA
true or false: the TCA cycle is also known as the Krebs cycle
true
the TCA cycle is named after Hans Krebs, who elucidated this pathway in the 1930s. It’s also called the citric acids cycle
true or false: if TCA cycle intermediates are constantly removed for biosynthesis and not replaced, the cycle will eventually stop
true
the cycle is catalytic — intermediated must be regenerated. if oxaloacetate and other intermediates are depleted without replenishment, the cycle cannot continue. this is why anaplerotic reactions are essential.
true or false: the TCA cycle produces a net gain of carbon-containing compounds
false
the cycle takes in a 2-carbon acetyl group and releases 2 CO2 molecules, with not net gain in carbon-containing intermediates. the intermediates are regenerated, not accumulated
match the TCA cycle intermediate with its primary biosynthesis use:
Citrate
α-ketoglutarate
succinyl-CoA
Oxaloacetate
citrate for fatty acids, α-ketoglutarate for amino acids (via glutamate), succinyl-CoA for heme, and oxaloacetate for amino acids (via aspartate).
the TCA BLANK slows when energy BLANK is BLANK
cycle/charge/high
when the energy charge (ATP/ADP ratio) is high, the cell has sufficient energy and the TCA cycle slows through allosteric inhibition of key regulatory enzymes.
a patient has a thiamine (vitamin B1) deficiency. which step would be most directly affected?
A. Citrate synthase
B. α-Ketoglutarate dehydrogenase
C. Isocitrate dehydrogenase
D. Succinate dehydrogenase
B. α-ketoglutarate dehydrogenase
which enzymes require TPP as a cofactor? think about the large multienzyme complexes in metabolism and what cofactors they need for decarboxylation reactions
A metabolic inhibitor specifically blocks succinate dehydrogenase in the TCA cycle. What would be the most immediate observable affect?
A. complete shutdown of the entire TCA cycle
B. accumulation of succinate and reduced FADH2 production
C. increased production of NADH
D. reversal of the TCA cycle direction
B. accumulation of succinate and reduced FADH2 production
blocking succinate dehydrogenase would cause succinate to accumulate and prevent FAD reduction to FADH2 at this step. the cycle would slow but not necessarily stop completely
cancer cells often exhibit altered TCA cycle function. which statement best describes the Warburg effect?
A. Cancer cells cannot perform glycolysis
B. Cancer cells rely heavily on the TCA cycle for energy
C. Cancer cells preferentially convert pyruvate to lactate (fermentation) even when oxygen is available
D. Cancer cells cannot use oxygen
C. cancer cells preferentially convert pyruvate to lactate (fermentation) even when oxygen is available
the Warburg effect describes how cancer cells favor glycolysis and lactate production even in the presence of oxygen, rather than fully oxidizing glucose through the TCA cycle and oxidative phosphorylation
a researcher measures very high levels of succinate in a tumor sample. what is the most likely explanation?
A. The tumor has increased TCA cycle activity
B. The tumor has a deficiency in succinate dehydrogenase
C. The tumor is using succinate as its primary fuel
D. The tumor cannot produce NADH
B. the tumor has a deficiency in succinate dehydrogenase
succinate dehydrogenase (SDH) deficiency is found in some tumors and leads to succinate accumulation. SDH is also complex II of the electron transport chain, so its loss affects both the TCA cycle and respiration
in a cell with high acetyl-CoA levels but low oxaloacetate levels, how would you predict pyruvate carboxylase activity will adapt?
A. Change to very low activity
B. Change to very high activity
C. No change in activity
D. The enzyme would be completely inhibited
B. change to very high activity
pyruvate carboxylase is activated by acetyl-CoA. High acetyl-CoA signals that more oxaloacetate is needed to condense with acetyl-CoA in the first step of the TCA cycle, so the enzyme is activated
a metabolic poison specifically inhibits α-ketoglutarate dehydrogenase. what would be the most immediate metabolic consequence?
A. Complete shutdown of the TCA cycle
B. Accumulation of α-ketoglutarate and reduced NADH production
C. Increased fatty acid synthesis
D. Reversal of the TCA cycle
B. accumulation of α-ketoglutarate and reduced NADH production
inhibiting α-ketoglutarate dehydrogenase blocks the conversion of α-ketoglutarate to succinyl-CoA, causing α-ketoglutarate accumulation and reducing NADH production downstream.
Match the regulatory molecule with its effect on isocitrate dehydrogenase
ATP
Ca2+
NADH
ADP
inhibitor
activator
inhibitor
activator
ATP and NADH (products indicating energy sufficiency) inhibit the enzyme, while ADP and Ca2+ (signals of energy demand, especially in muscle) activate it.
true or false: a patient with a genetic deficiency in citrate synthase (step 1, which makes citrate) would likely have more severe metabolic problems than a patient with a deficiency in malate dehydrogenase (step 8, which makes oxaloacetate)
true
citrate synthase catalyzes the first step that brings acetyl-CoA into the cycle. without it, acetyl-CoA cannot enter and the entire cycle fails. malate dehydrogenase, while important, is the last step that regenerates oxaloacetate. other pathways can potentially supply oxaloacetate through anaplerotic reactions.
true or false: if all anaplerotic reactions were blocked, increasing the amount of acetyl-CoA entering the cycle would solve the resulting metabolic problems.
false
acetyl-CoA needs oxaloacetate (a 4-carbon intermediate) to condense with to form citrate. If intermediates are being removed for biosynthesis and cannot be replenished by anaplerotic reactions, adding more acetyl-CoA won’t help — you’ll still lack oxaloacetate
per acetyl-CoA, the TCA cycle produced BLANK NADH, BLANK FADH2 and BLANK ATP/GTP.
3/1/1
a biochemist is designing a drug to selectively target cancer cells that exhibit the Warburg effect. Which approach would be most logical?
A. Inhibit all glycolytic enzymes
B. Activate the TCA cycle in all cells
C. Target metabolic vulnerabilities specific to high-glycolysis cells
D. Block oxygen delivery to tumors
C. target metabolic vulnerabilities specific to high-glycolysis cells
since Warburg-effect cancer cells rely heavily on glycolysis, they may have unique metabolic vulnerabilities or dependencies that normal cells (which can use oxidative phosphorylation) don’t have. selective targeting exploits these differences.
An organism is discovered that can survive without oxygen but still runs a complete TCA cycle. what must be true about this organism
A. It doesn't need NAD⁺ or FAD
B. It has alternative ways to reoxidize NADH and FADH₂
C. It runs the TCA cycle in reverse
D. It doesn't produce NADH or FADH₂
B. it has alternative ways to reoxidize NADH and FADH2
the TCA cycle itself doesn’t require oxygen directly, but the NADH and FADH2 produced must be reoxidized for the cycle to continue. this organism must use alternative electron acceptors (like nitrate, sulfate, or fumarate) instead of oxygen.
true or false: supplying excess succinyl-CoA will overcome arsenite inhibition of α-ketoglutarate dehydrogenase and normalize NADH production during the TCA cycle
false
arsenite inhibits α-ketoglutarate dehydrogenase, preventing the conversion of α-ketoglutarate to succinyl-CoA. adding succinyl-CoA bypasses the blocked step but doesn’t restore the NADH production hat normally occurs at that specific enzymatic reaction
in isolated muscle mitochondria you add a high Ca2+ and measure a sudden rise in NADH production. which regulatory event best explains this?
A. Ca²⁺ inhibits citrate synthase
B. Ca²⁺ activates isocitrate dehydrogenase
C. Ca²⁺ converts FADH₂ back to FAD
D. Ca²⁺ stimulates ATP synthase directly
B. Ca2+ activates isocitrate dehydrogenase
Ca2+ is a key activator of isocitrate dehydrogenase in muscle mitochondria. during muscle contraction, Ca2+ levels rise, activating this enzyme and accelerating the TCA cycle to meet increased energy demands
a novel drug specifically increases the Km of synthase for oxaloacetate three-fold. which observation would best confirm that the drug slows overall oxidative phosphorylation in intact cells?
A. Accumulation of α-ketoglutarate
B. Rise in intracellular citrate levels
C. Drop in NADH/NAD⁺ ratio
D. Increased PEP carboxykinase activity
C. Drop in NADH/NAD+ ratio
increasing Km means citrate synthesis has lower affinity for oxaloacetate, slowing the cycle entry point. thi reduces TCA cycle glux, decreasing NADH production and thys lowering the NADH/NAD+ ratio
true or false: if a cell diverts large amounts of citrate to fatty-acid synthesis, simultaneously knocking out both pyruvate carboxylase and PEP carboxykinase will still allow normal TCA-cycle-dependent ATP production
false
pyruvate carboxylase and PEP carboxykinase are both involved in anaplerotic reactions that replenish oxaloacetate. if citrate is being removed and these enzymes are knocked out, oxaloacetate cannot be replenished, and the cycle will slow or stop.
a sprinter’s skeletal-muscle mitochondria experience a brief Ca2+ spike just as ADP levels rise. which single enzyme activation best explains the sudden jump in NADH production?
A. Citrate synthase
B. α-Ketoglutarate dehydrogenase
C. Isocitrate dehydrogenase
D. Malate dehydrogenase
C. isocitrate dehydrogenase
isocitrate dehydrogenase is activated by both Ca2+ and ADP. in muscle, Ca2+ spikes during contraction coordinate energy demand with supply, and IDH is the key control point for accelerating the cycle
your engineered yeast exports large amounts of citrate for fatty-acid synthesis. which co-engineering step keeps oxidative ATP output intact?
A. Knock out isocitrate dehydrogenase
B. Over-express pyruvate carboxylase
C. Delete succinyl-CoA synthetase
D. Block β-oxidation (fatty acid degradation)
B. over-express pyruvate carboxylase
exporting citrate removes a key intermediate from the TCA cycle. over-expressing pyruvate carboxylase increases oxaloacetate production (an anaplerotic reaction), replenishing the cycle intermediates and maintaining ATP production
during prolonged fasting, muscle protein breakdown increases. how does this connect to TCA cycle function?
A. Amino acids from protein can feed into the TCA cycle as intermediates
B. Protein breakdown shuts down the TCA cycle
C. Amino acids inhibit TCA cycle enzymes
D. Protein breakdown only affects glycolysis
A. Amino acids from protein can feed into the TCA cycle as intermediates
during fasting, amino acids from muscle protein degradation are deaminated, and their carbon skeletons enter the TCA cycle as intermediates (e.g., glutamate → α-ketoglutarate, aspartate → oxaloacetate). this supports gluconeogenesis and energy production
a person consumes a meal high in glucose. which sequence best describes the metabolic flow?
A. Glucose → lactate → TCA cycle
B. Glucose → pyruvate → PDH → acetyl-CoA → TCA cycle
C. Glucose → fatty acids → TCA cycle
D. Glucose directly enters the TCA cycle
glucose → pyruvate → PDH → acetyl-CoA → TCA cycle
under aerobic conditions with glucose available, glycolysis converts glucose to pyruvate, which is then decarboxylated by pyruvate dehydrogenase (PDH) to form acetyl-CoA, which enters the TCA cycle
Why doesn’t fatty acid oxidation, which produces acetyl CoA, contribute to net glucose synthesis in animals?
A. Fatty acids cannot be broken down
B. Acetyl-CoA from fatty acids cannot be converted to net oxaloacetate due to TCA cycle stoichiometry
C. Animals lack the enzymes for fatty acid oxidation
D. Fatty acids inhibit gluconeogenesis
B. acetyl-CoA from fatty acids cannot be converted to net oxaloacetate due to TCA cycle stoichiometry
for every 2 carbons entering the TCA cycle as acetyl-CoA, 2 carbons leave as CO2. there’s no net production of oxaloacetate (needed for gluconeogenesis) from acetyl-CoA. animals lack the glyoxylate cycle that plants use to accomplish this.