Chapter 12 - Reactions of biologically important compounds
1/80
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
81 Terms
Homopolymer
Has an amine and a carboxyl group
Has the same type of repeating monomer all throughout (with two different types of functional groups at the end)
Many biological polymers are built this way
Has an amine and carboxyl functional group
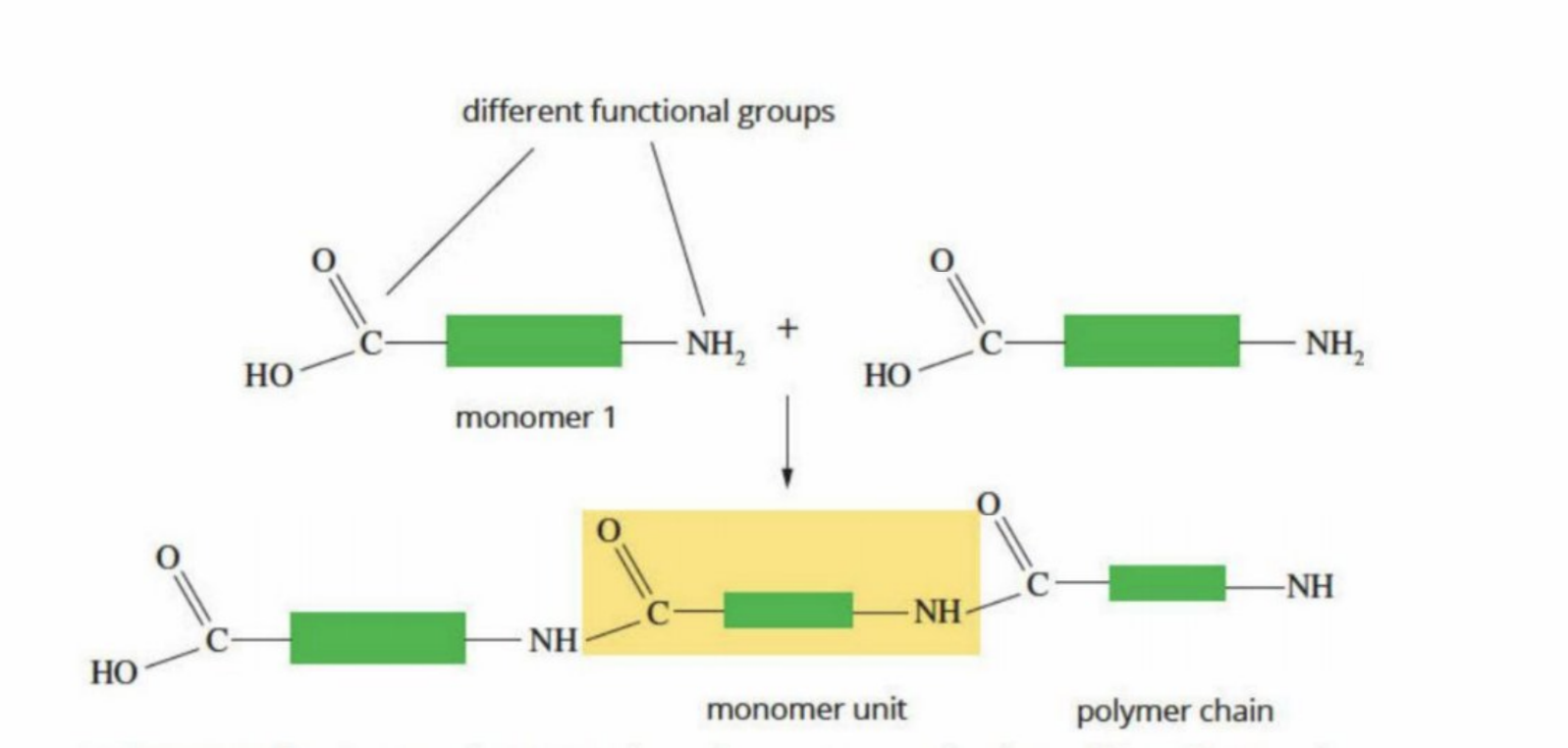
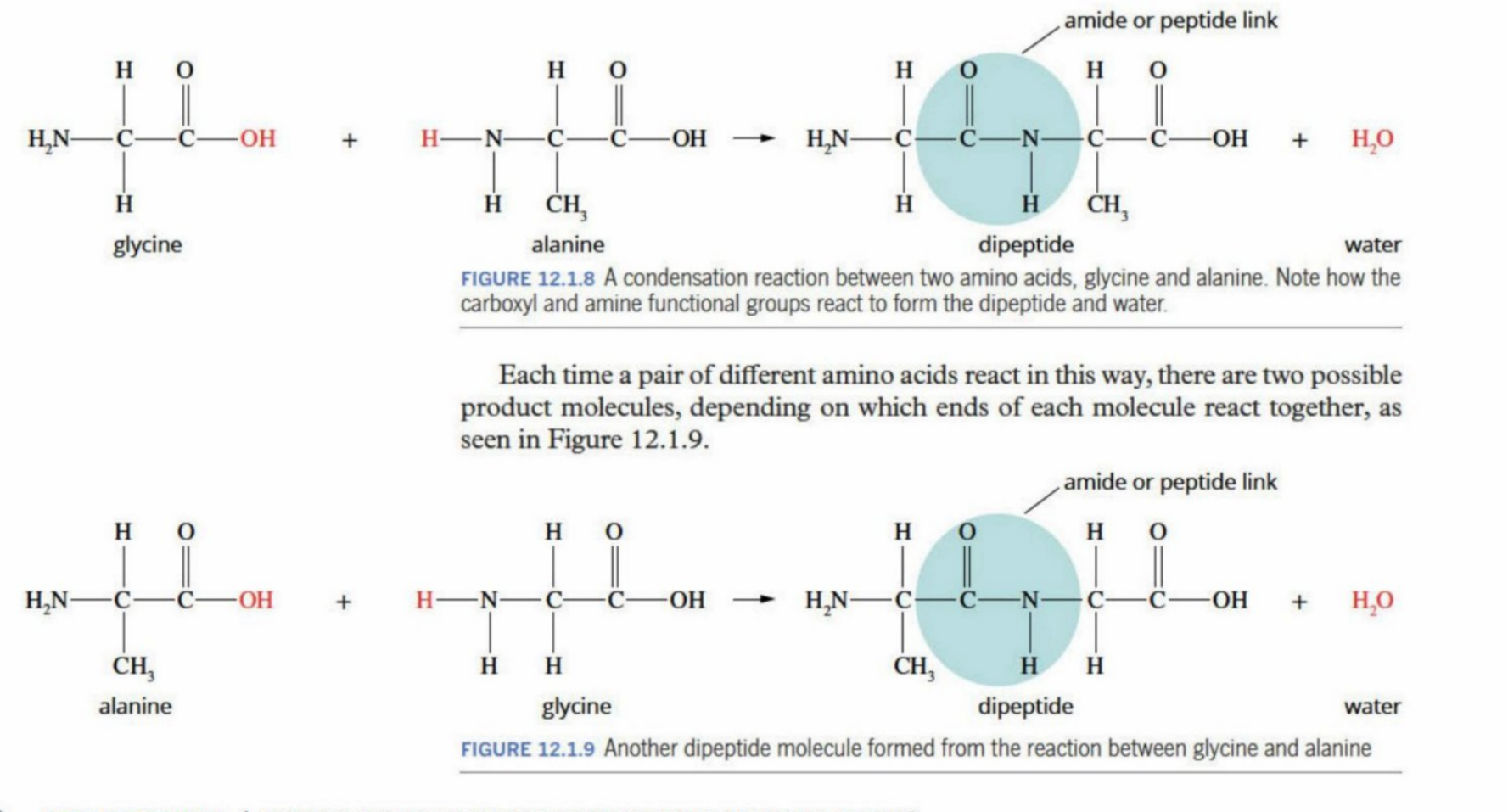
Condensation Reactions (In terms of the body)
Proteins, Carbohydrates, Lipids - all are formed by a condensation reactions
Heteropolymer
Two or more different kinds of monomers are used within the same polymer
They each have different functional groups
One monomer is a diol (two hydroxyl groups - can be internal (OH inside the carbon chain) or terminal (OH outside the carbon chain)
2nd monomer has two carboxylic acids - two carboxylic acid functional groups
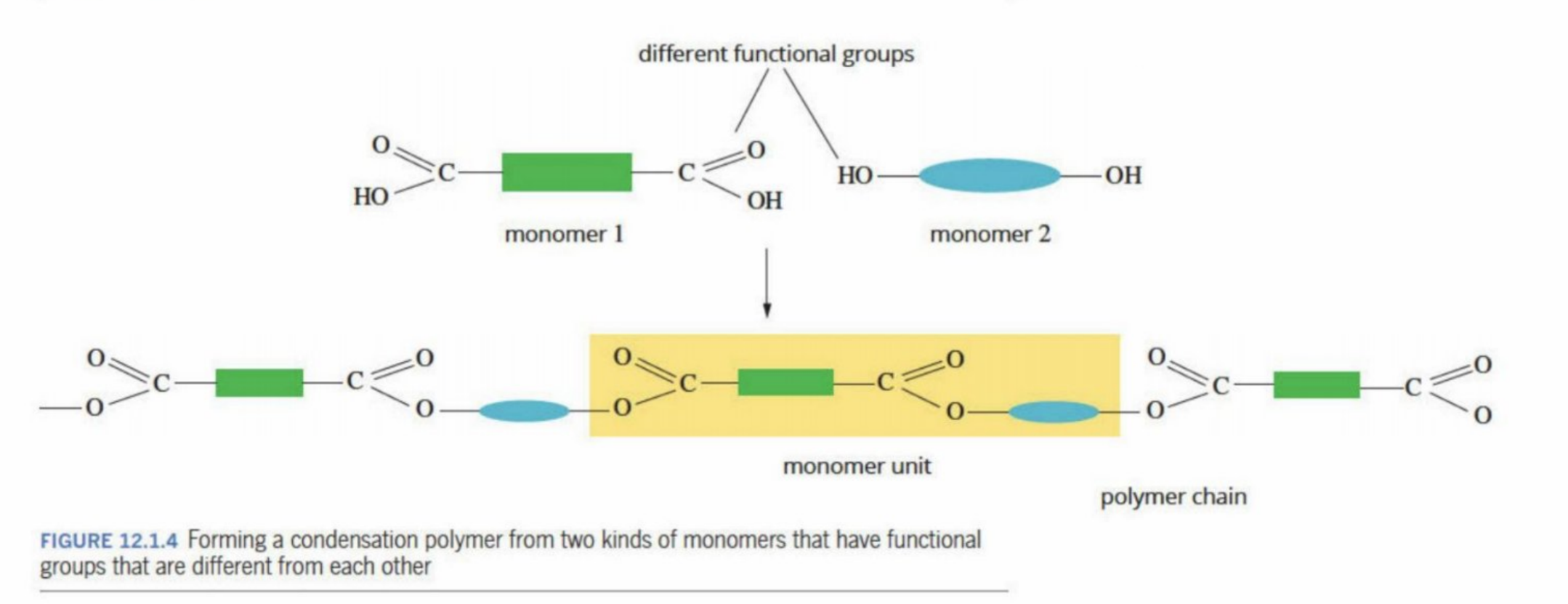
Condensation Reaction By-products
Mainly water
Ammonia, hydrogen chloride and methanol - occasionally formed as byproducts of condensation reactions
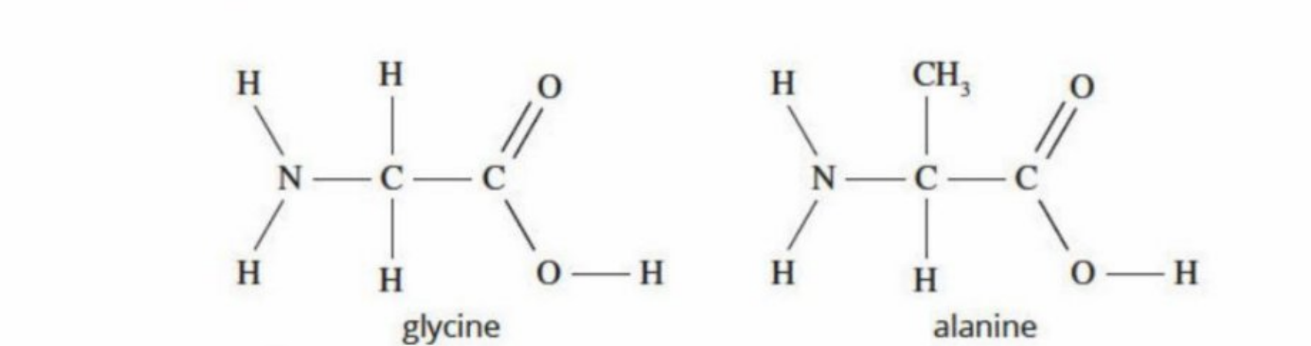
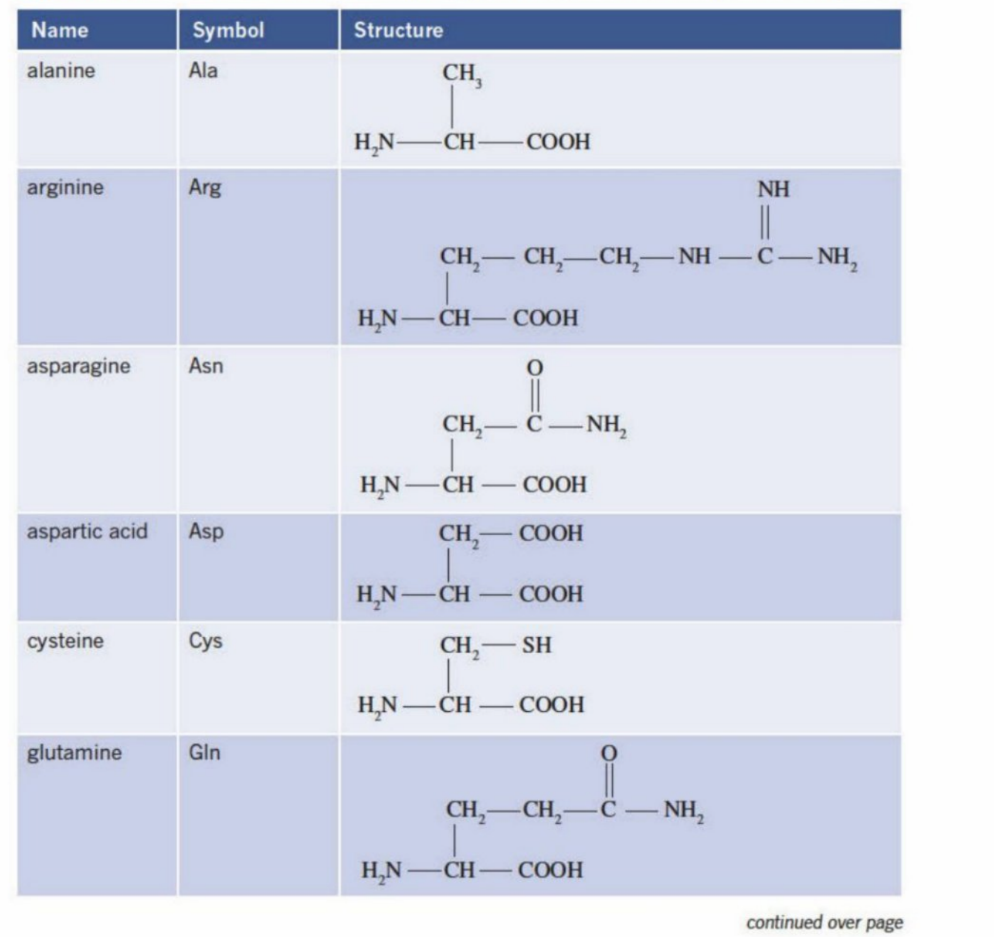
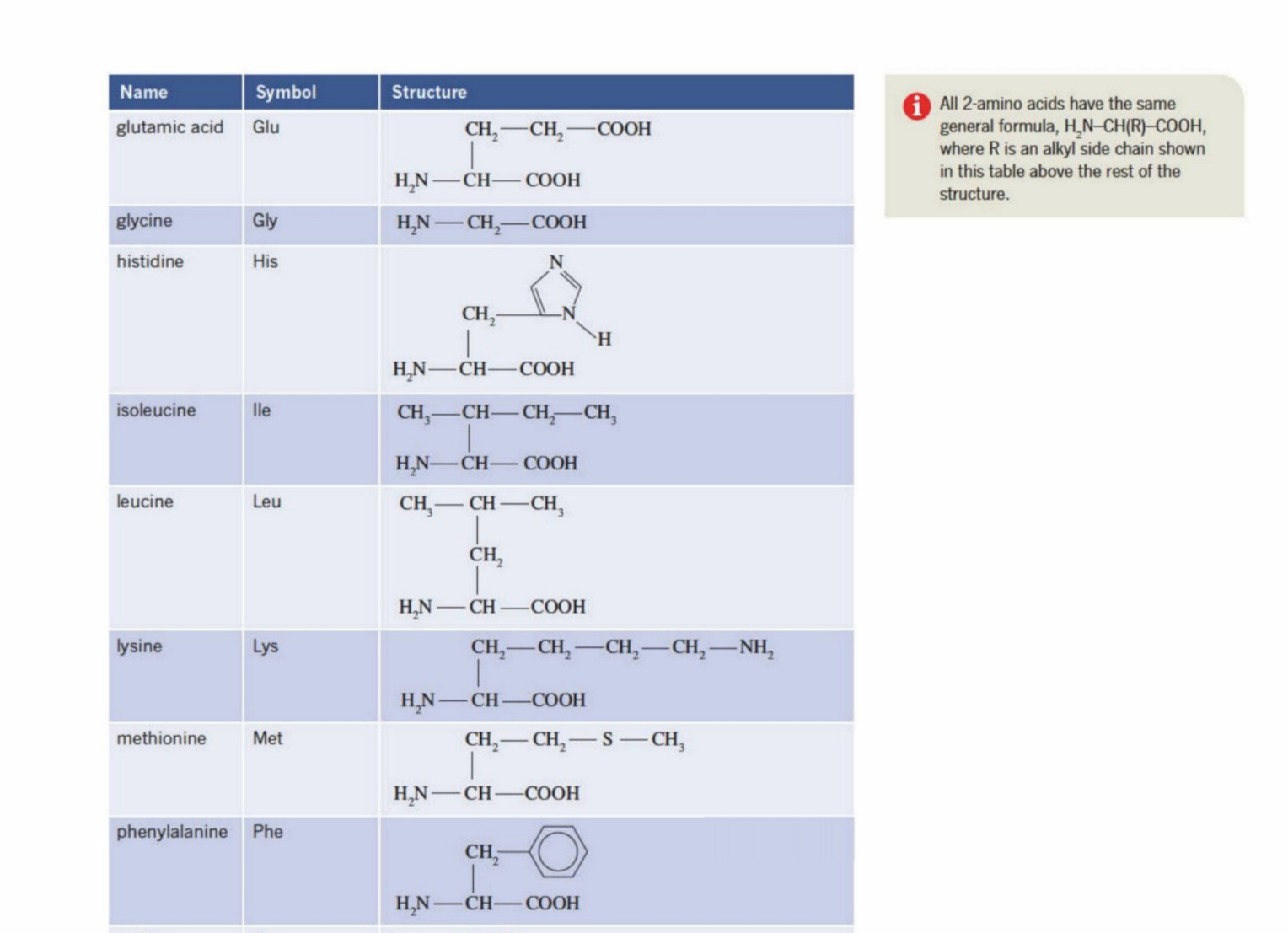
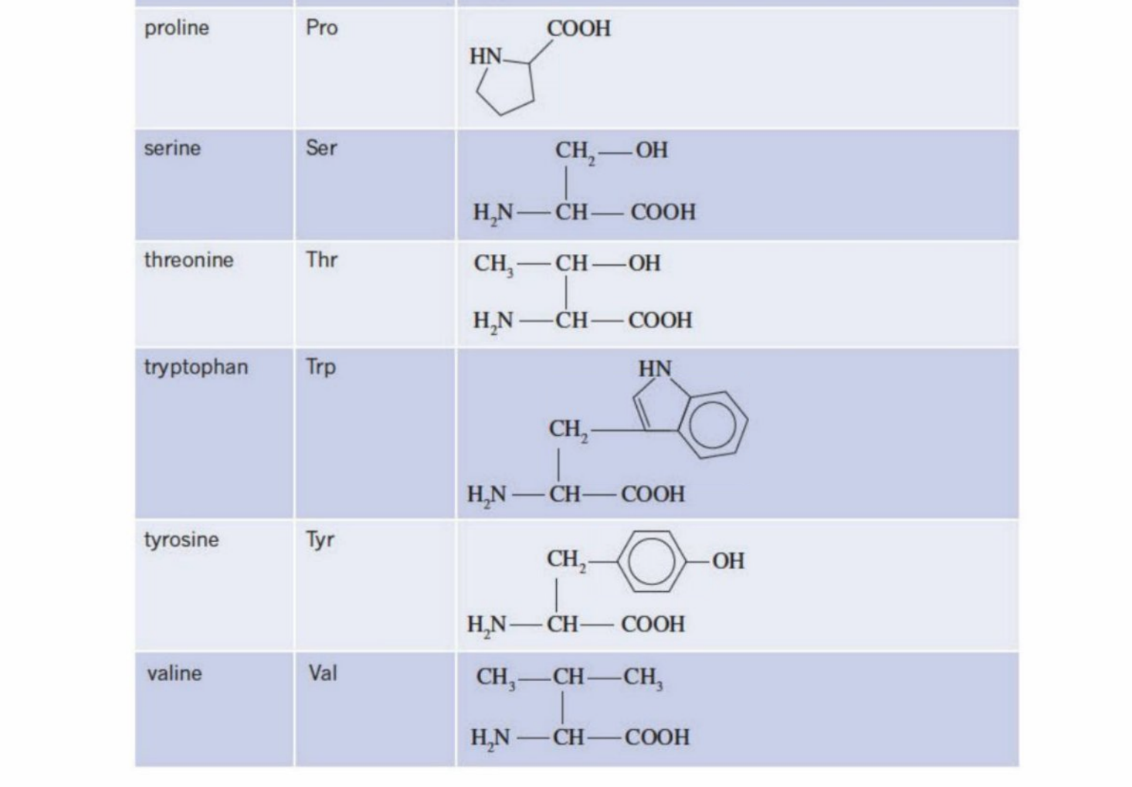
Glycine and Alanine (Two of the simplest amino acid structures)
Both have amine group and carboxyl group
General Formula Of Amino Acids (Add picture here)
H2N-CH(R)-COOH (amine group, carbon bonded with another chain, carboxyl group)
2-amino acids
The very next carbon after the carboxyl group is termed as the second carbon - 2nd carbon
The amine (amino) is bonded to the second carbon atom - hence is called (2-amino acid)
is also called a-amino acids
How properties of the side chain (R) affect the amino acid itself - Unclear
Definition of R group:
The basic structure of an amino acid is:
H₂N–CH(R)–COOHThe R group is the variable part attached to the central carbon (α-carbon).
How R group affects the amino acid:
Polarity:
Polar R groups → hydrophilic (water-loving) → often found on the outside of proteins.
Non-polar R groups → hydrophobic (water-fearing) → often buried inside protein structures.
Charge:
Acidic R groups (e.g., –COOH) → negatively charged at physiological pH.
Basic R groups (e.g., –NH₂) → positively charged at physiological pH.
Size and shape:
Bulky R groups (like tryptophan or phenylalanine) can affect protein folding.
Small R groups (like glycine) allow flexibility in the protein chain.
Special properties:
Some R groups can form disulfide bonds (cysteine), hydrogen bonds (serine), or act as catalysts in enzymes (histidine).
How amino acids are denoted
In three-letter abbreviations
e.g. Alanine - Ala
Glycine - Gly
Amino acids in body #1
Amino acids in body #2
Amino acids in body - #3
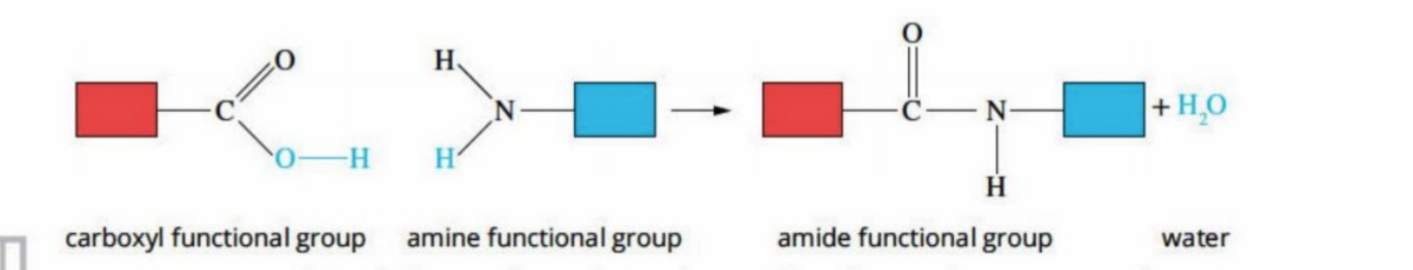
How protein is formed (dipeptide)
When two monomers (amino acids) bond, a peptide link /amide is made(as two monomers are involved)
Called dipeptide
Amide is made (review) - CONH
Water is pushed off
How protein is formed (polypeptide)
When the process for a dipeptide occurs many times - over and over
Overall polymer is called polypeptide
Examples
The direction and the functional groups that interact dictate the product molecule made - can be different —→
Tripeptide
When three amino acids are formed
Where biological polymers are made between amino acids
In the ribosome - a biological synthesizing machine
One monomer unit = 4.2 million gmol-1
Synthesizing proteins in labs
The synthesis occurs across solid state catalysts
In 85-90 degrees Celsius
Can have protein tailor made in few hours
They add amino acids one-by-one - making it into a polypeptide
How polypeptides are named (Need more attention)
The first three letters named together
e.g. Ala-Glu-Gly-Cys-Val-Lys
N terminal
The side which has the amine group (NH2)
C terminal
The part which has the carboxyl group (COOH)
Insulin
Made up of 51 amino acid residues
Is formed through condensation polymer reaction
Has an N-terminal at the start (next to Phe - Phenylalanine), because it is not involved in the reaction
Has a C-terminal at the end (next to Thr - Threonine, because the COOH is not bonded to anything)
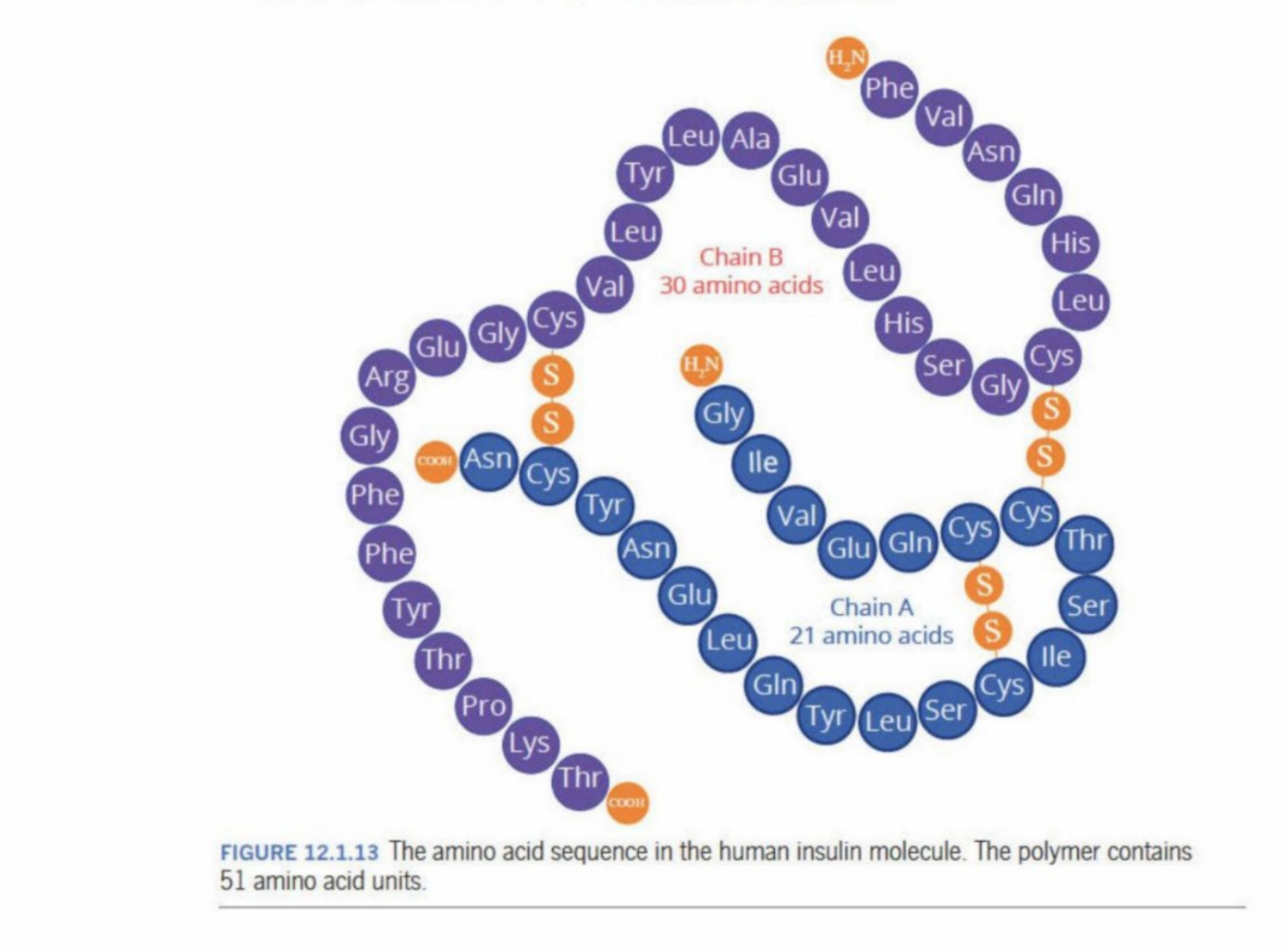
Sulfurs within the insulin
S - sulphur atoms
Are part of cytosine
When the sulphides form, they create a disulphide bridge/disulphide bond
Act like “molecular staples” and hold the insulin molecule in shape
Main structrual component of all plants
Polymer cellulose
Belongs to carbohydrates
Cabrohydrate Formula
Cx(H20)y - x and y are whole numbers
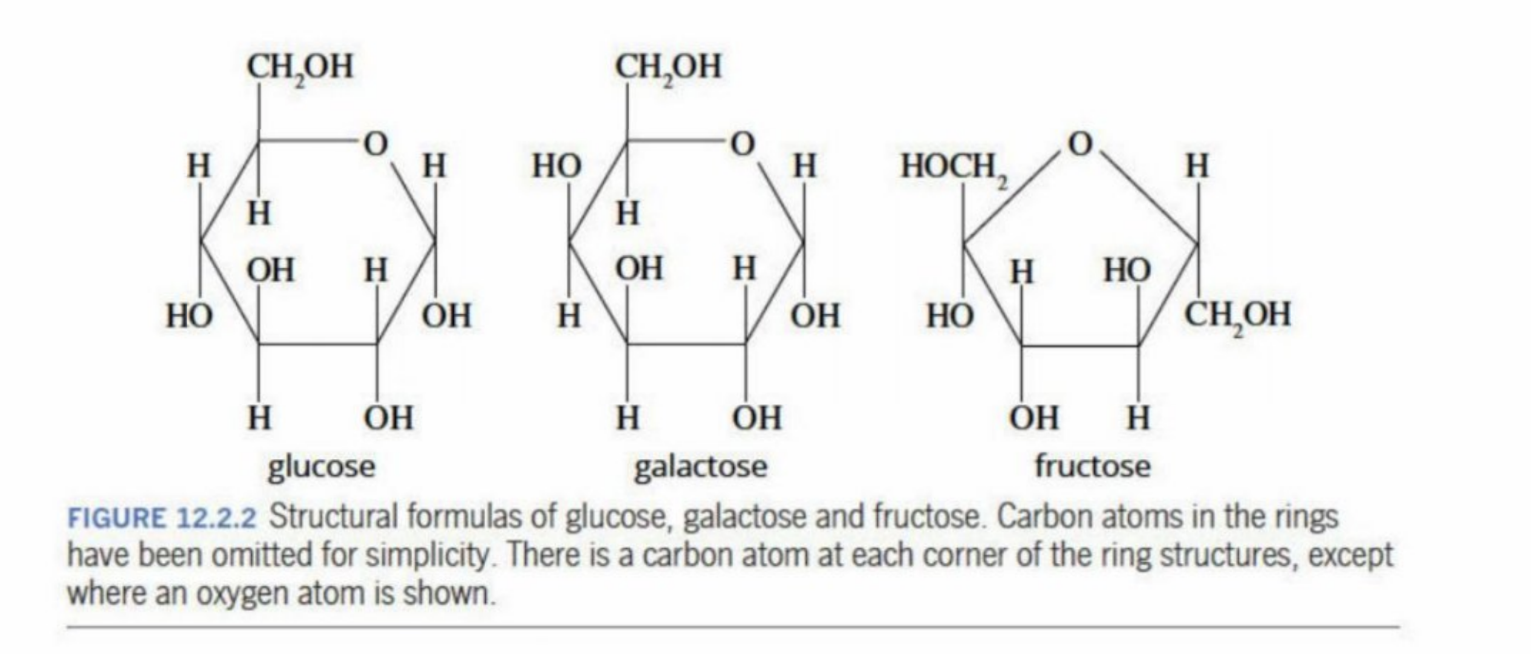
Monosaccharides
Smallest carbohydrates
White, sweet-tasting and highly soluble in water
Glucose, Galactose, Fructose (Isomers of one another)
Are all isomers of one another, (e.g. the OH group in glucose and galactose are different)
All are C6H12O6
All have polar hydroxyl groups - they can make hydrogen bonds with water - are highly soluble
Glucose
In all living things
Juice of fruits, sap of plants
Blood and tissue of animals
Product of photosynthesis - key energy source in most forms of life
Fructose
In many fruit juices and honey
Main role in body is an energy source
Most common sugar in fruit, especially berries
Galactose
Not found free in nature (Cells use galactose as a building block, not mainly as a fuel.
Free galactose can be toxic in high amounts, so the body tightly controls it.
It’s more chemically stable and biologically useful when attached to other molecules.)
Bonded with larger compounds (e.g. lactose- the main sugar in milk)
Disaccharides
Carbohydrates created by the reaction between two monosaccharide molecules
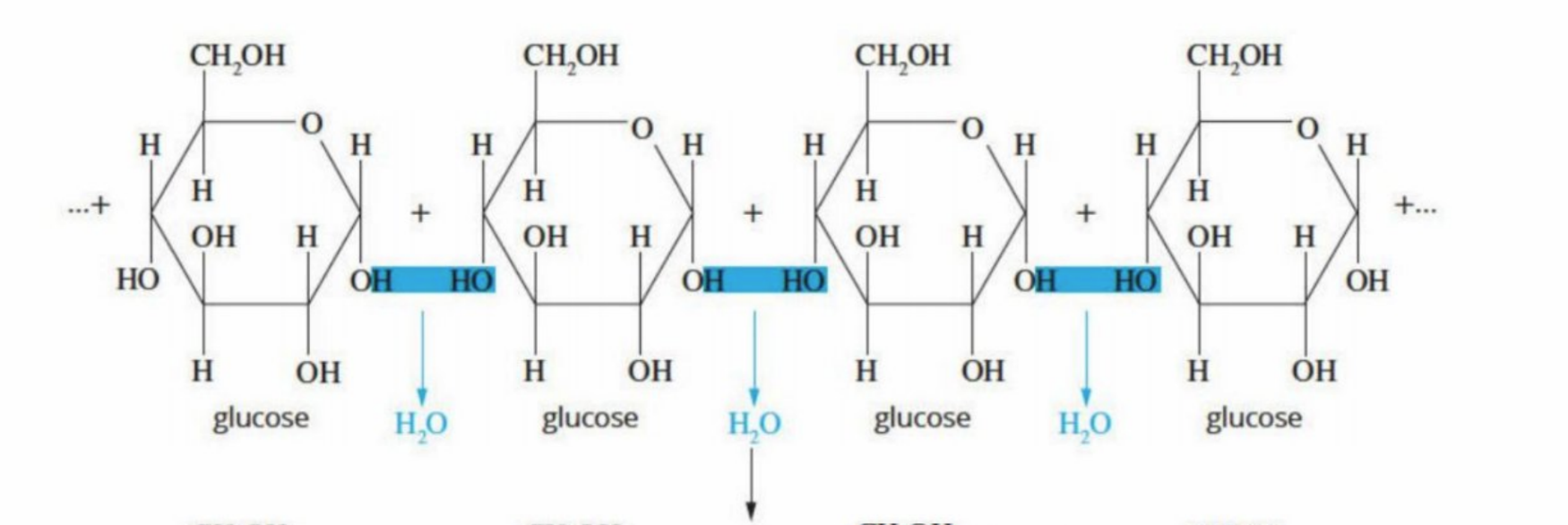
Are bonded through a condensation reaction - water is ejected
Leaves behind a glycosidic link/ether functional group
Glucose + Glucose = Maltose
Glucose + Fructose = Sucrose
Ether functional group/Glycosidic Link (Insert Visual Representation Here)
The link found between monosaccharide units in a carbohydrate
R-O-R’ - formula - Oxygen is between two carbon chains
Maltose
Used in beer fermentation (as it has a high concentration of glucose, which is used to make ethanol)
Derived from barley
Sucrose
Popular sweetener
High concentrations in sugarcane and sugar beet
Polysaccharides
Have a lot of monosaccharides (more than disaccharides - like a long chain)
Regulated by enzymes in the body
Are generally insoluble in water (unclear) + have no taste
Polysaccharides (Visual Representation)
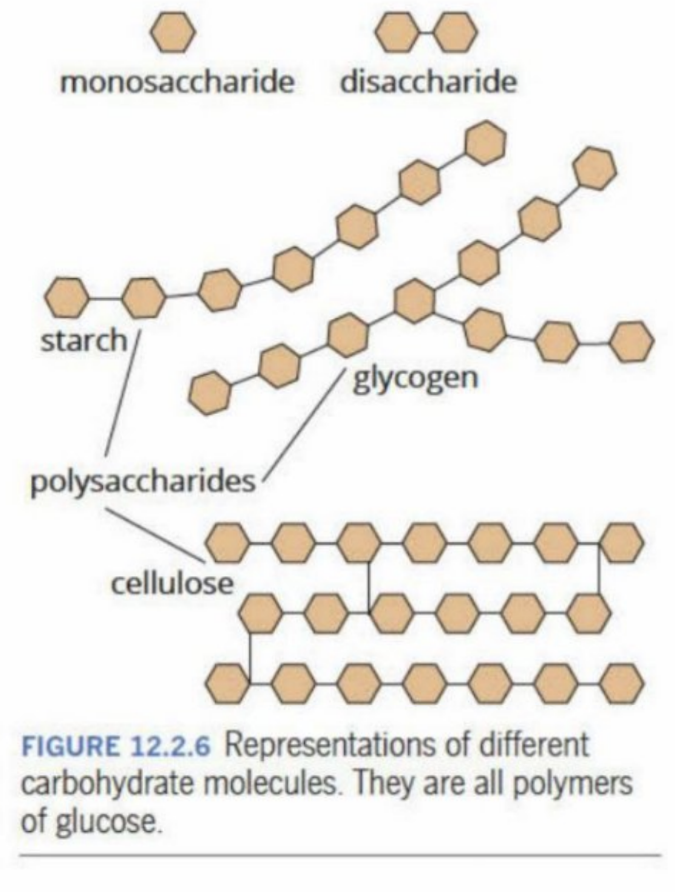
Starch (In relation to polysaccharides)
Polysaccharides are formed through condensation of glucose (monosaccharide)
Is called amylose - the overall structure (polysaccharide of glucose structures)
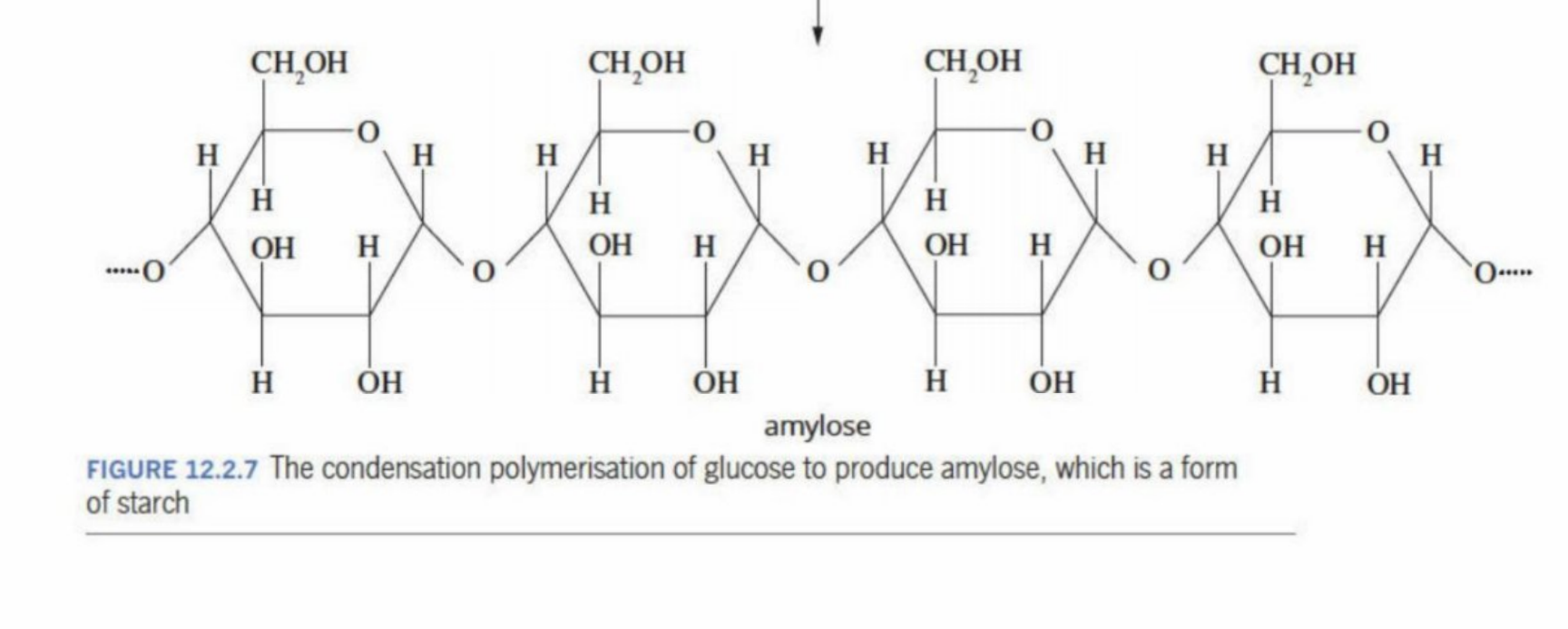
Amylose
1. What amylose is
Amylose is a type of starch.
Starch is how plants store energy.
Amylose is made only of glucose molecules (the same sugar in honey and fruit).
2. How it’s built (structure)
Glucose molecules are joined together by α-1,4 glycosidic bonds (a type of ether bond).
The chain is mostly straight (unbranched).
Because of hydrogen bonding, the chain twists into a coil (helix). - the coil restricts OH groups to be exposed to water, thus preventing solubility
Think of it like: a long string of beads twisted into a spring.
3. Properties
Insoluble in water because:
It’s very large,
The –OH groups are bonded to each other (hydrogen bonds), not free to bond with water.
Can form a gel when heated with water (like when you cook rice or make jelly).
Compact energy storage → plants can store a lot of energy in a small space.
4. Biological role
Energy storage in plants.
Slowly digested → gives long-lasting energy for humans and animals.
Condensation to become a starch
Amylose (Full Structure)
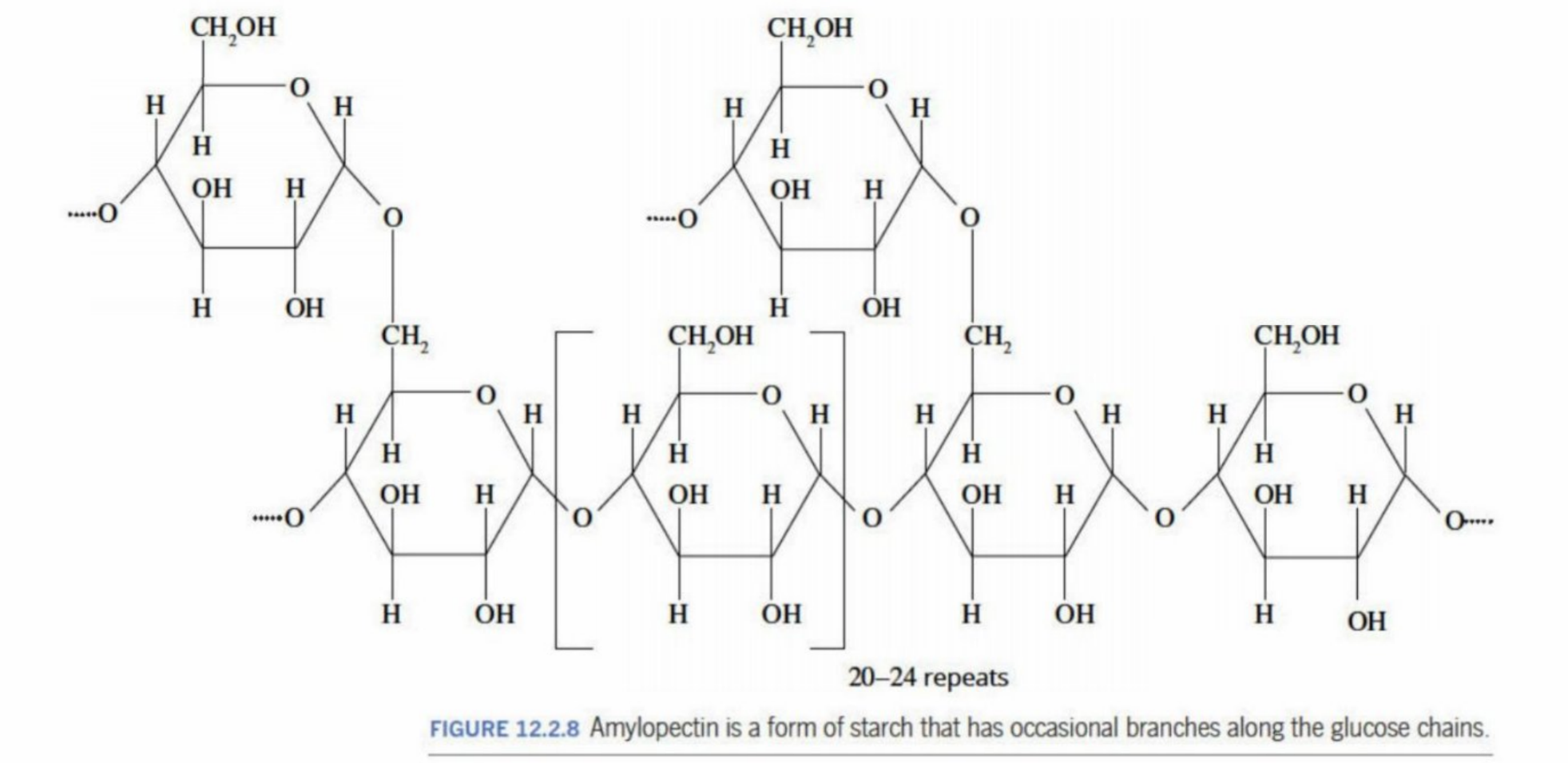
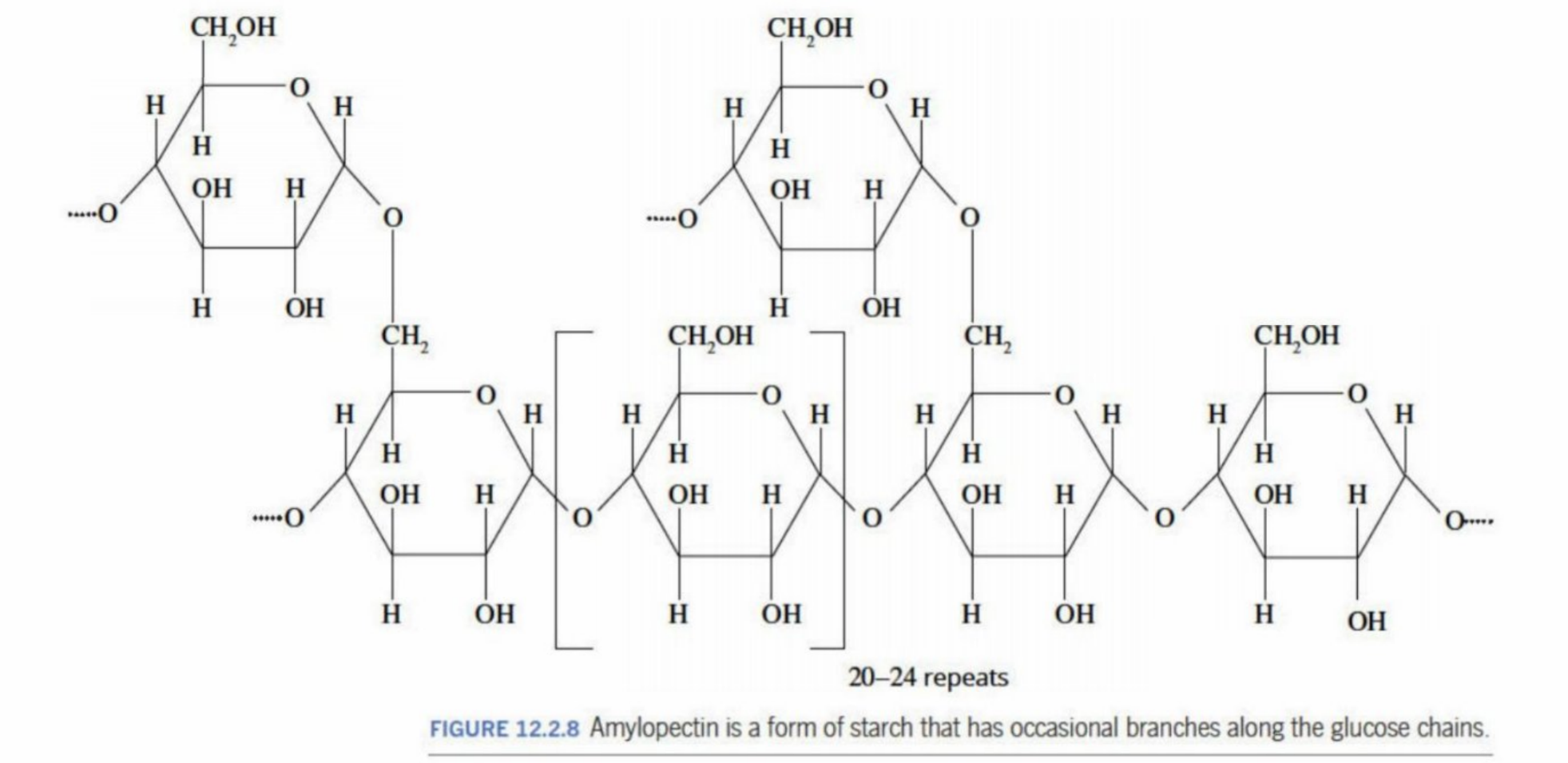
Amylopectin
A second form of starch
Can occur if the hydroxyl groups undergo condensation in different positions than from the ones in starch
Occasional branching occurs
Branching of the polymer restricts coiling (like starch) and allows more -OH groups to be exposed, interacting with water - is more soluble than starch
Amylopectin (Structure)
Glycogen
Similar structure to Amylopectin (but is even more branched - has very high solubility) - polymerization of only glucose - all of these are only polymerizations of glucose
Is formed due to excess glucose and is stored in the liver or in muscle tissue (as fat)
Energy can be taken from these stores, and then be used in cellular respiration - used for energy
Glycogen Structure
Lipids
Are fats and oils - in meat, fish, dairy products and eggs
Are a source of the unsaturated fatty acids
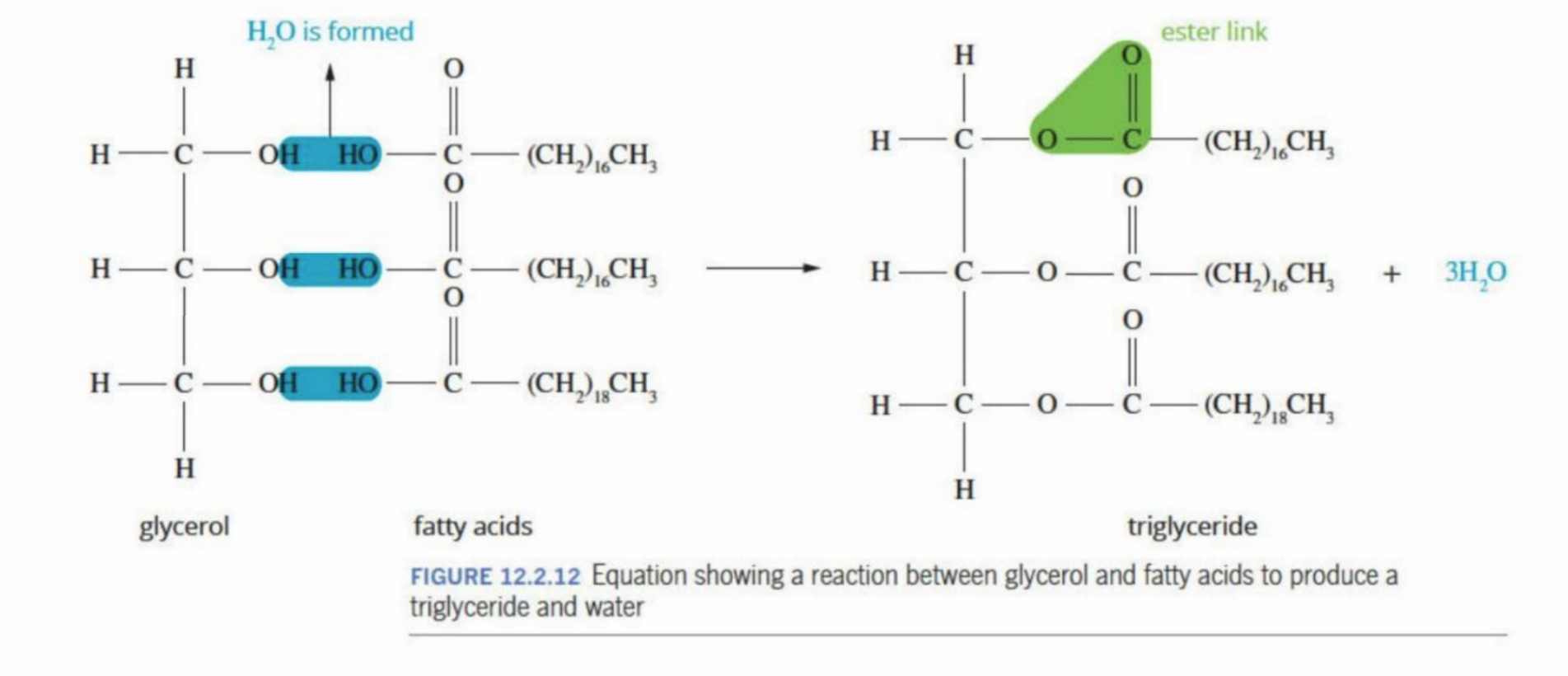
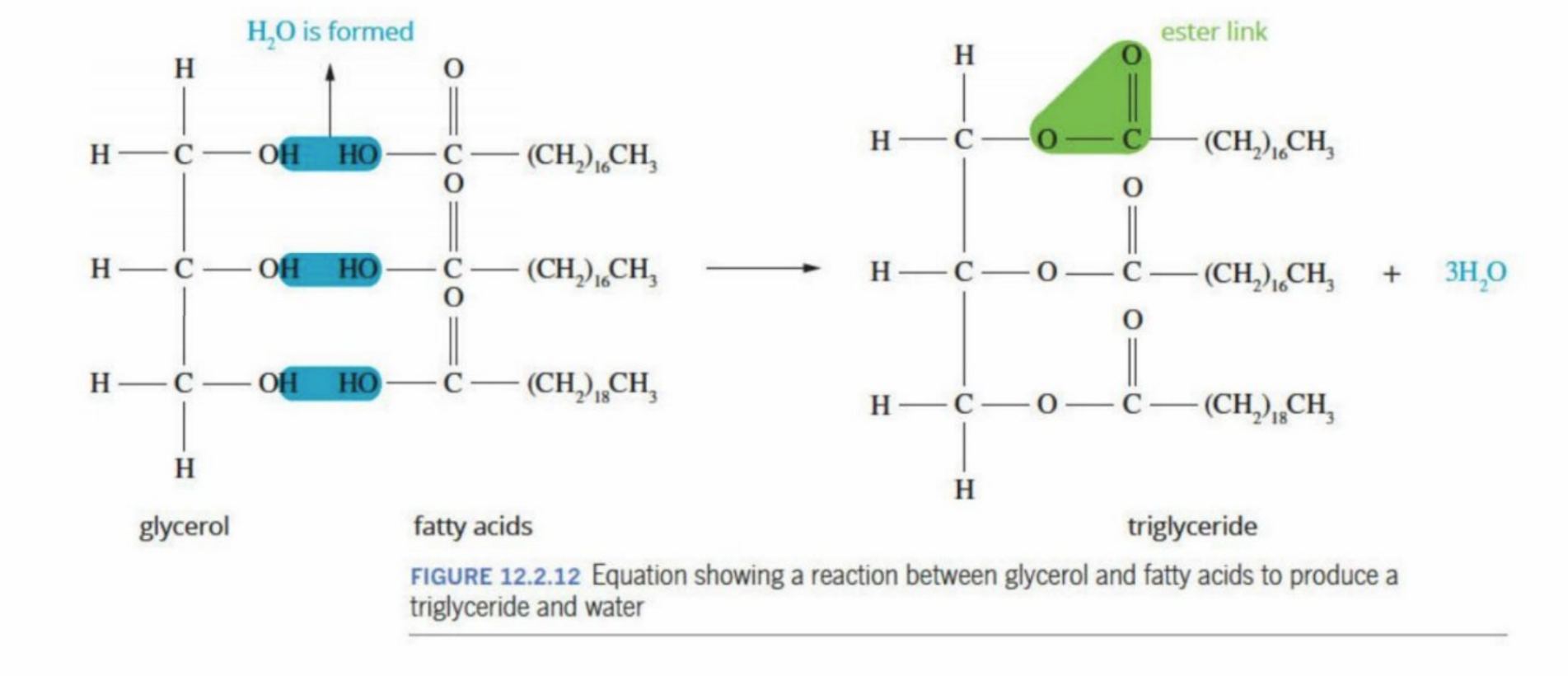
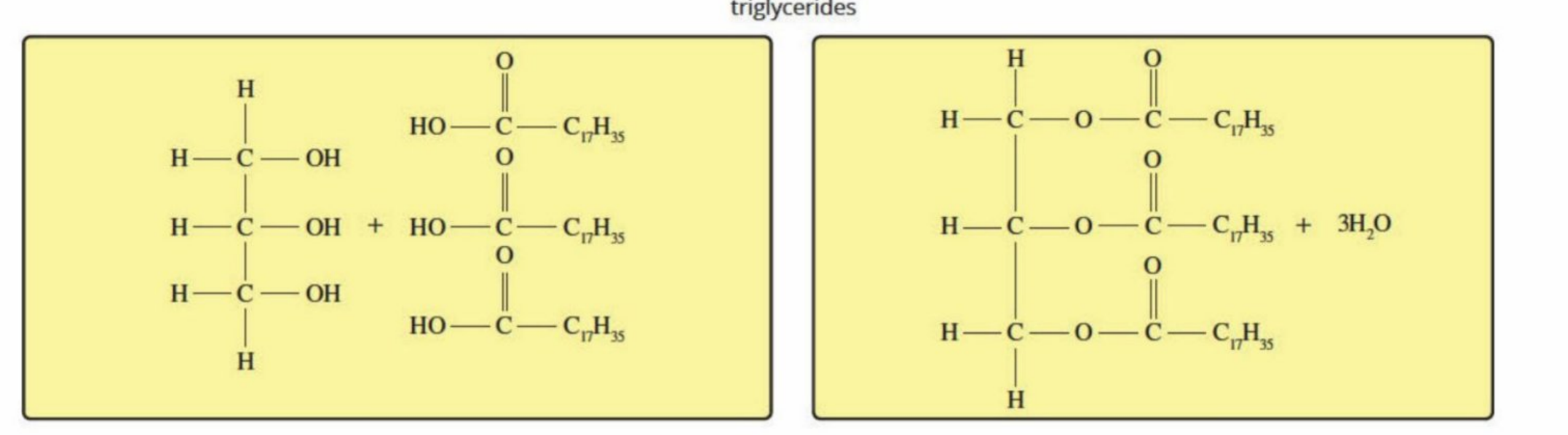
Triglycerides
Large, non-polar structures (fat)
fats - solids
oils are liquids
Unable to form hydrogen bonds with water (is non-polar) - are insoluble in water and are immiscible (cannot mix in water)
How triglcerides are formed (revision)
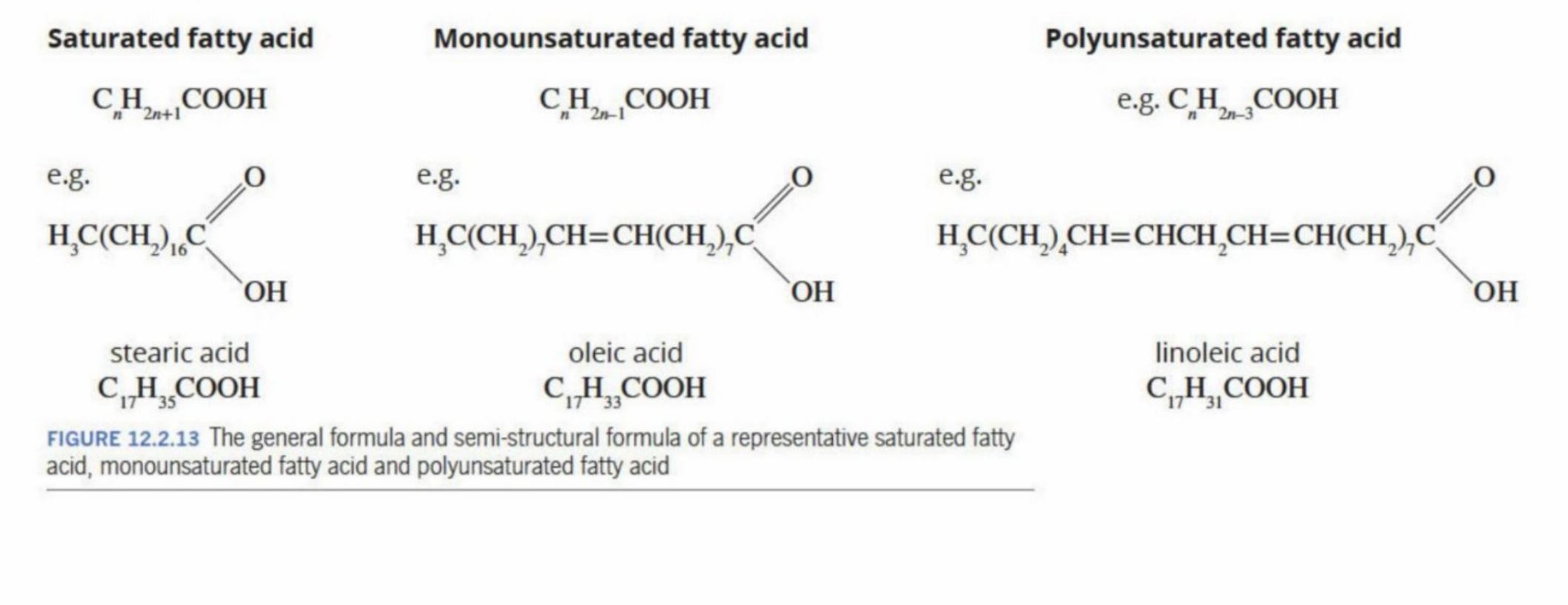
Monosatruated fatty acid
Has one C=C bond
“kink” in the bonds”
reduces close packing - lower boiling point
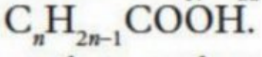
Saturated fatty acids
No kinks
Have close packing and are more crystalline - higher boiling point
Polyunsaturated fatty acids
More than one C=C bond
Multiple “kinks” in the carbon chain
Is least crystalline - and therefore less packing
Lower boiling point
Examples
Hydrolysis
Reducing molecules into smaller individual atoms
Used to rebuild into new substances that are required for the body
The deconstructed atoms are transported to different parts of the body to become reconstructed
Two main chemical reactions to reconstruct and break down nutrient molecules
Condensation and hydrolysis
Hydrolytic Reactions
Reactions involving hydrolysis
Condensation
Joining of two molecules with the elimination of water
Enzyme
A biological catalyst used to accelerate the rate of reaction in condensation and hydrolysis reactions
Some only catalyze one particular reaction, or only work is a specific functional group is present
Condensation Reaction Examples
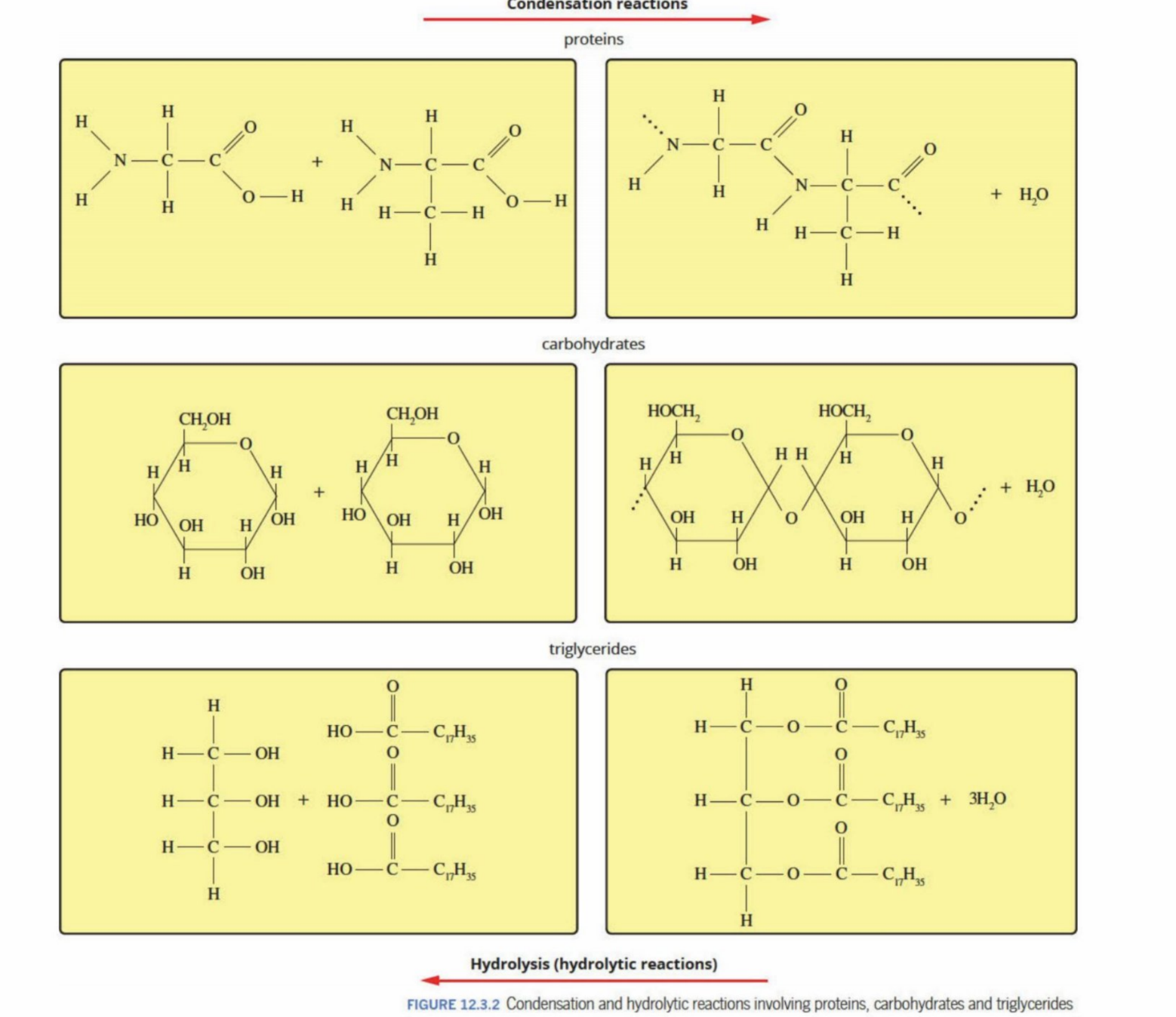
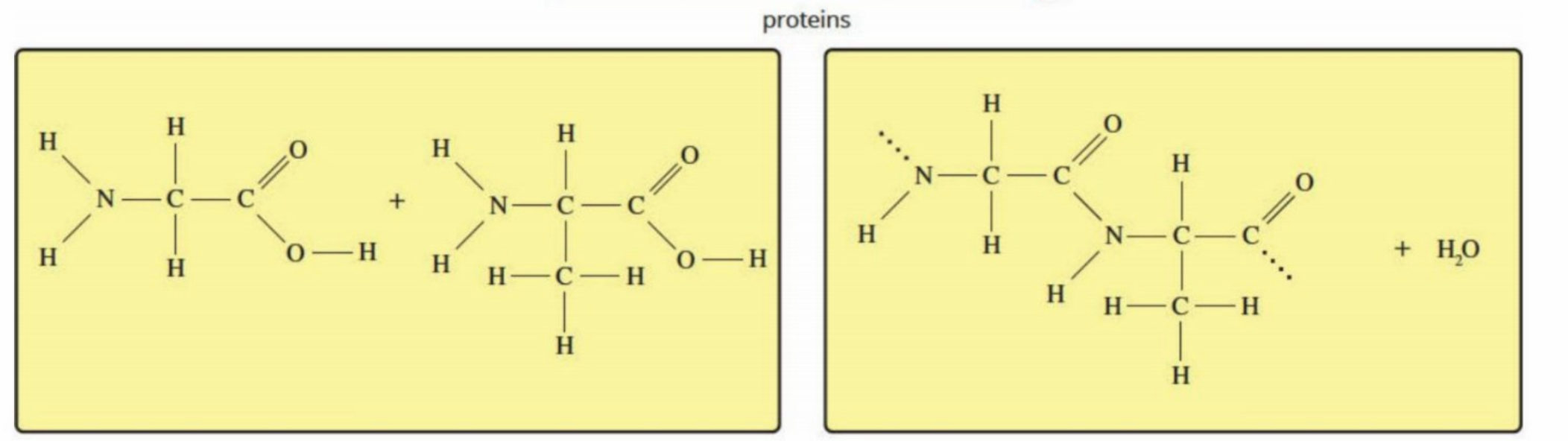
Proteins (Hydrolysis and condensation)
Hydrolysis: The amide bond breaks and the amino acids are separated into their own parts
They undergo condensation later to form new proteins once they are transported to cells
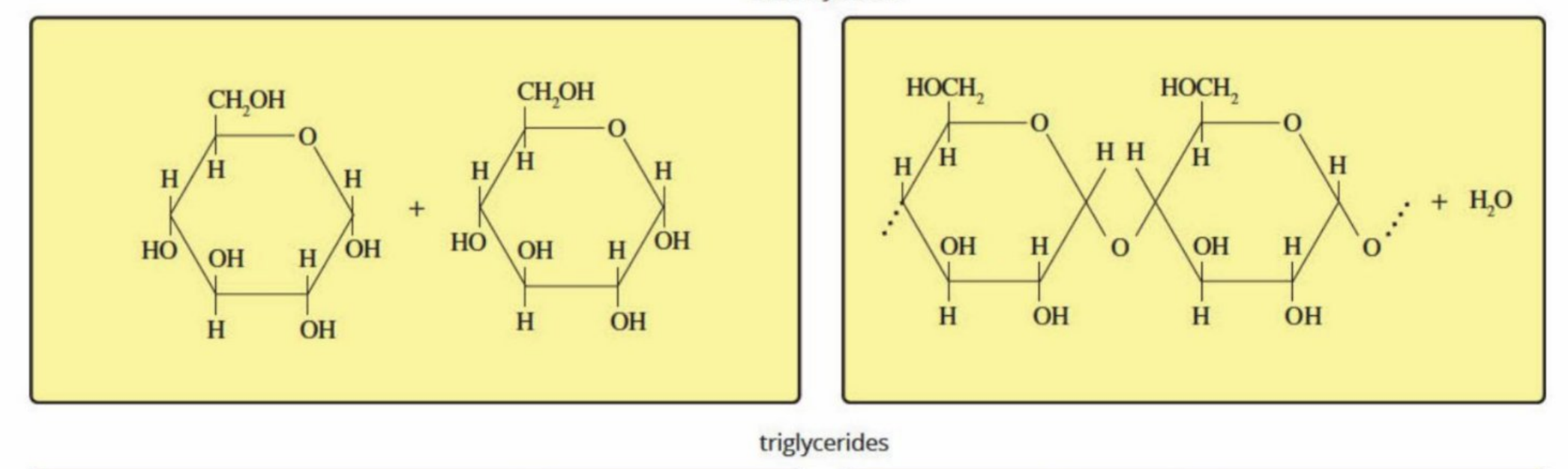
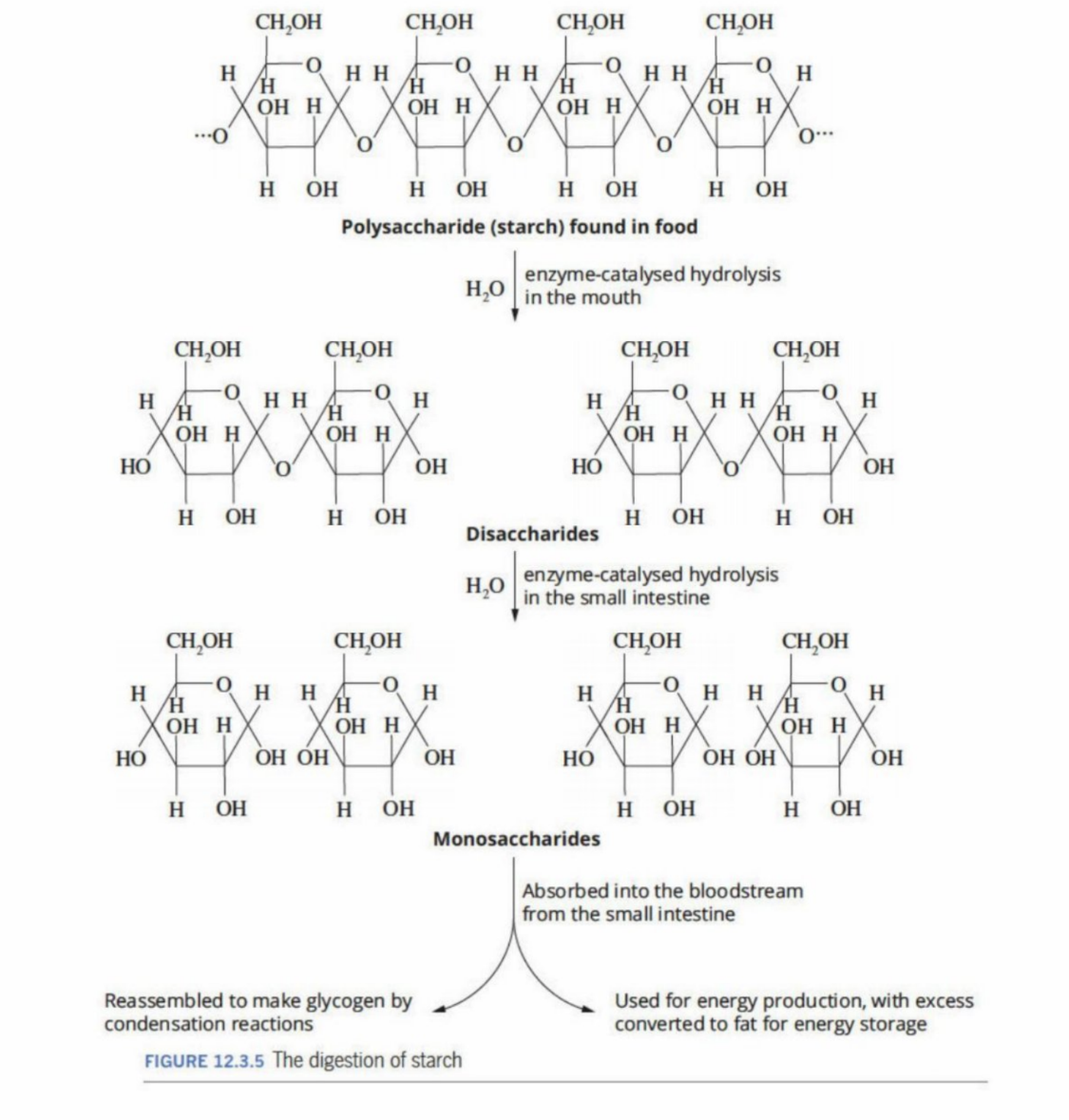
Polysaccharides (Hydrolysis and condensation)
Hydrolysis: Hydrolyzed into monosaccharides and disaccharides by hydrolysis of ether bond
Condensation: Are converted back into polysaccharides such as glycogen for energy stores
Triglycerides (Hydrolysis and condensation)
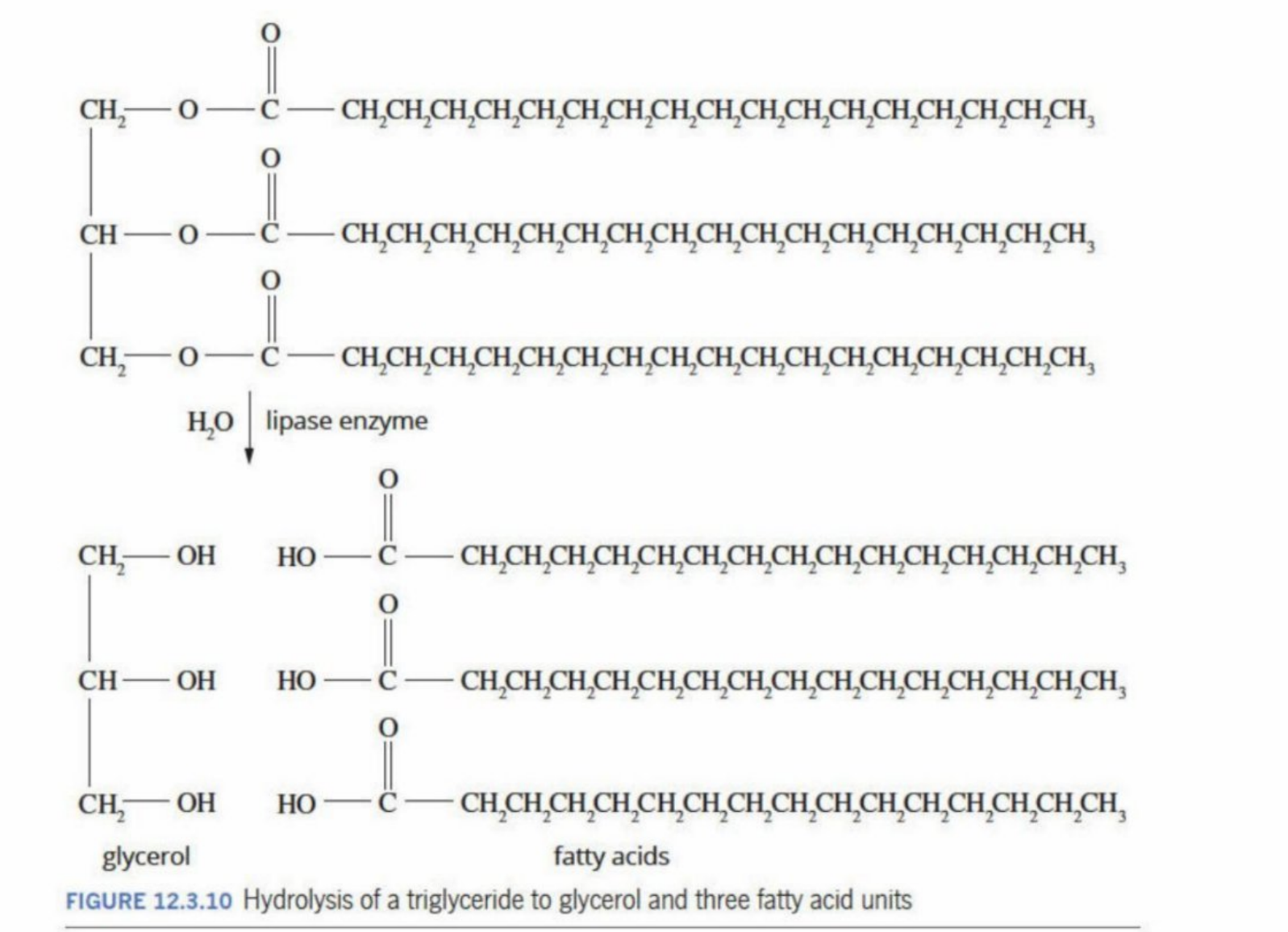
Hydrolysis: Triglycerides are hydrolyzed back down to glycerol and fatty acids - through hydrolysis of ester bond
Condensation: Can be converted back to triglycerides to produce/use energy in cells
Summary (Hydrolysis and Condensation)
Metabolism
The overarching term that describes the hydrolysis and condensation of molecules within the body
Condensation (Endothermic)
Energy is required to fixate the bonds in between molecules
Hydrolysis (Exothermic)
Energy is released as the bonds are broken in the formation of smaller molecules
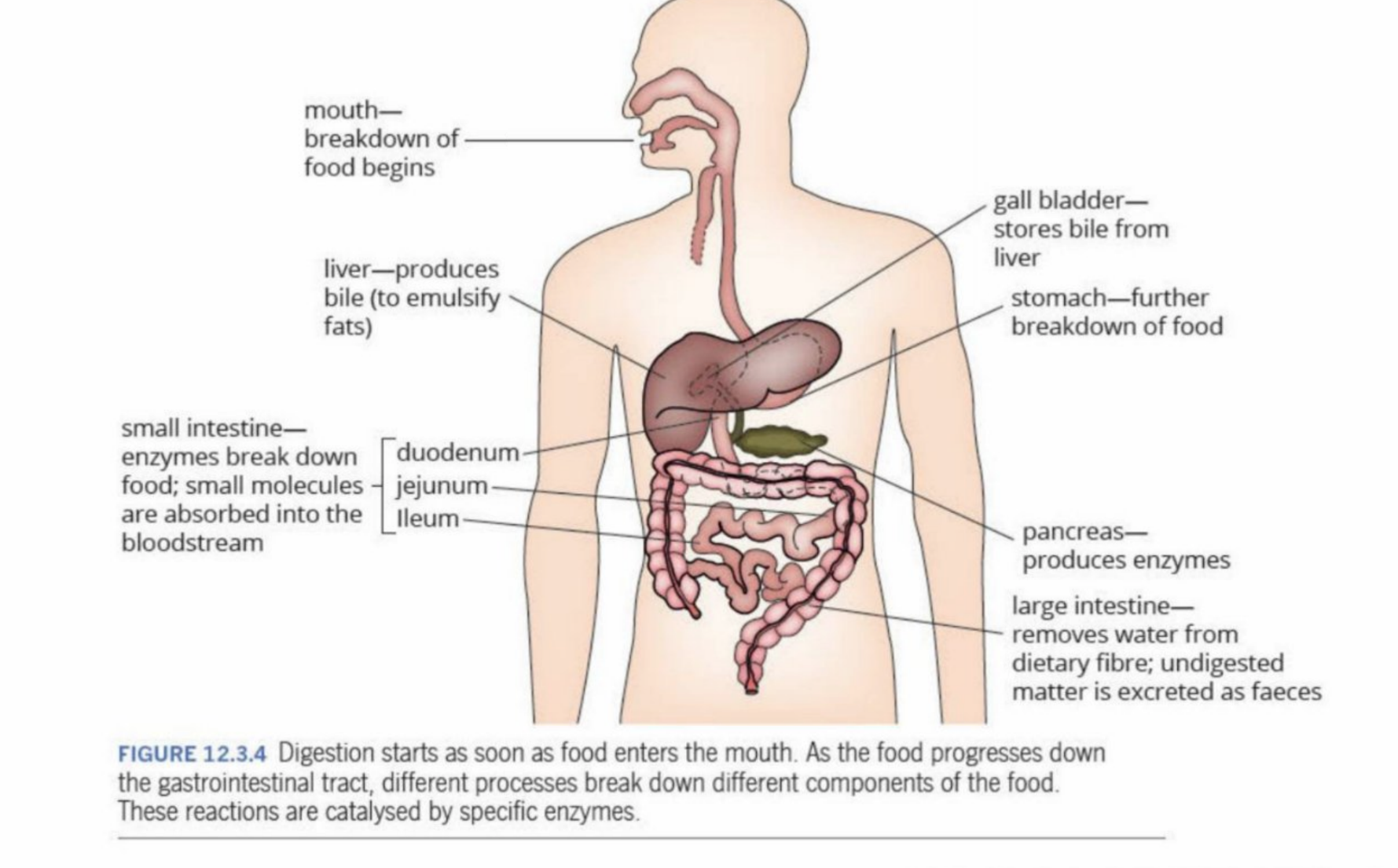
Digestion
1st phase of metabolism
Has many different enzymes throughout the sustem to break down the components of food
Overview of Digestion
Hydrolysis of carbohydrates in body through digestion
1) Chewing - Increases surface area of the food, allowing greater contact with the enzyme amylase (found in saliva) - breaks carbohydrates down into smaller disaccharides (two monosaccharides bonded together)
2) The disaccharides (e.g. maltose) - broken down in smaller monosaccharides by specific digestive enzymes
What does saliva do
Hydrolyses the starch within the food to maltose (a disaccharide - is also found within the lactate of milk - is also sweet)
Maltase
An enzyme is small intestine - aids in digestion and hydrolysis
Maltase (enzyme) hydrolyzes maltose(from the mouth) into glucose
Visual (Of Hydrolysis)
Why are glucose molecules highly soluble
Has a lot of hydroxyl groups —→ can easily make hydrogen bonds with the water present in the blood
Can easily be transported to different parts of the body
Are used to produce energy through respiration
Others are used to make energy storages (e.g. glycogen)
Cellulose Hydrolysis
Cellulose—→ is known as dietary fiber or roughage
Humans lack cellulase (the enzyme required to hydrolyze the cellulose)
Though we cannot digest cellulose, it aids in digestion and can prevent constipation, hemorrhoids and colon cancer
How does cellulose allow better digestion (Revew)
a) Adds bulk to your poop
Fibre soaks up water and makes your poop bigger and softer.
Bigger poop moves through your intestines faster, so you don’t get constipated.
b) Slows sugar absorption
Some fibres form a gel in your gut.
This makes sugar from food enter your blood slower, which is better for energy and blood sugar control.
c) Feeds good bacteria
Some fibre is eaten by gut bacteria.
These bacteria make healthy chemicals that help your intestines stay happy.
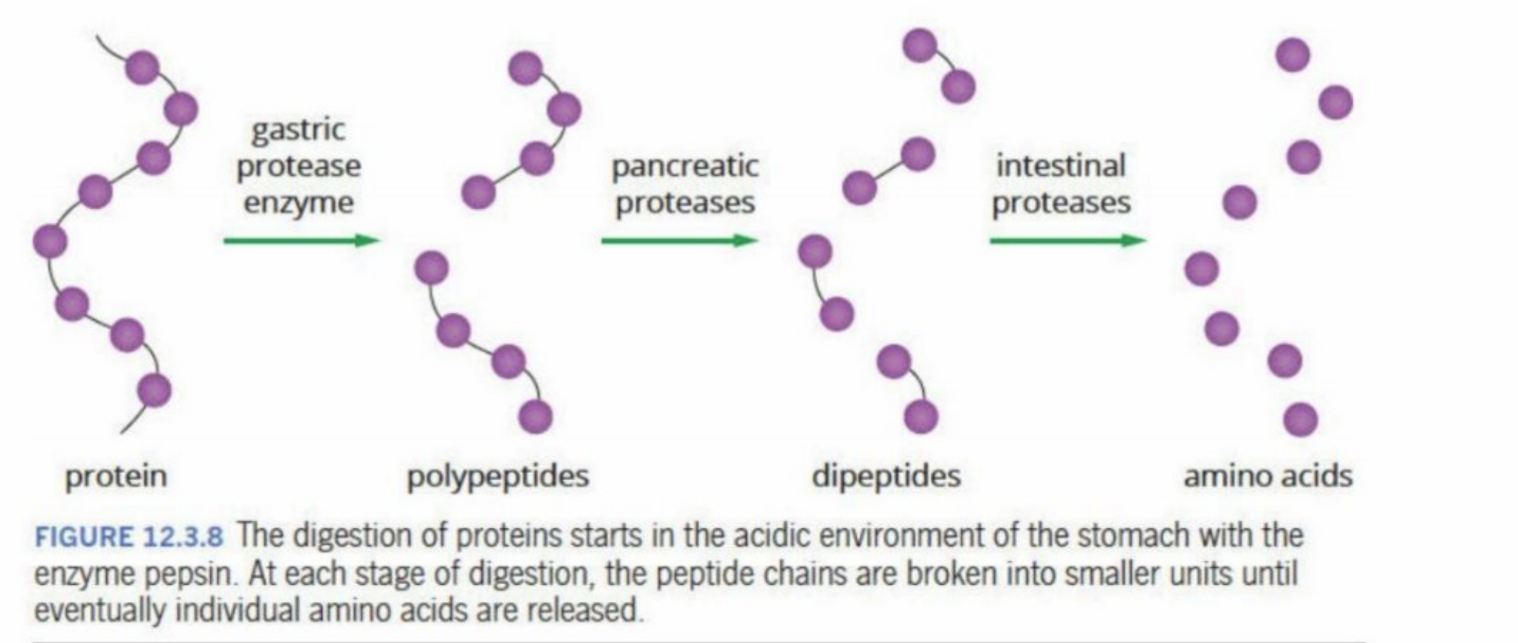
Hydrolysis Of Proteins
Happens in the stomach with the enzyme pepsin - activated by HCI
Pepsin breaks the protein into smaller polypeptides (smaller chains of amino acids)
Continue to break down into dipeptides (done with pancreatic protease) and then into amino acids (intestinal protease)
Protease
An enzyme that helps break down proteins
How HCI induced Pepsin (Unclear)
The stomach secretes pepsinogen (inactive form of pepsin).
HCl lowers the pH in the stomach and converts pepsinogen → active pepsin.
Pepsin then breaks proteins into smaller peptides.
Visual representation of protein being broken down
Stomach—→ Pancreas—→ Intestines
Hydrolysis of triglycerides
Triglycerides, as they are non-polar (insoluble in water) - cannot be hydrolyzed directly
Bile (from the small intestine) is released and breaks the large triglyceride into smaller droplets
This increases the surface area for the lipase to interact with more triglyceride molecules aiding in a quicker, more effective hydrolysis
Emulsion
The process of breaking down fats and spreading them into small droplets
Lipase
A lipase is an enzyme that breaks down fats (triglycerides) into glycerol and fatty acids.
From the pancreas
Catalyzes the hydrolysis of three of the ester bonds in the triglycerides - forms glycerol and the fatty acids
Hydrolysis in the presence of lipase
The glycerol backbone is seperated from the fatty acids
Note: The fatty acids can be different hydrocarbons (can have different length and size) - is represented by R, R’ and R’’ (can be the same structure) - represents each branch of the fatty acids