Combustion - 1st Sem Chemistry
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
Combustion
Exothermic chemical reaction between a substance (the fuel) and an oxidant (usually oxygen) that releases energy as heat and light
CₓHᵧ + O₂ → CO₂ + H₂O
State the format for a Combustion Equation:
CₙH₂ₙ₊₂
State the format for an alkane (fuel):
Bunsen burner

Barrel (Chimney)
Allows gas and air to mix before being ignited at the top.

Airhole
Openings that allow air to enter to mix with the gas.
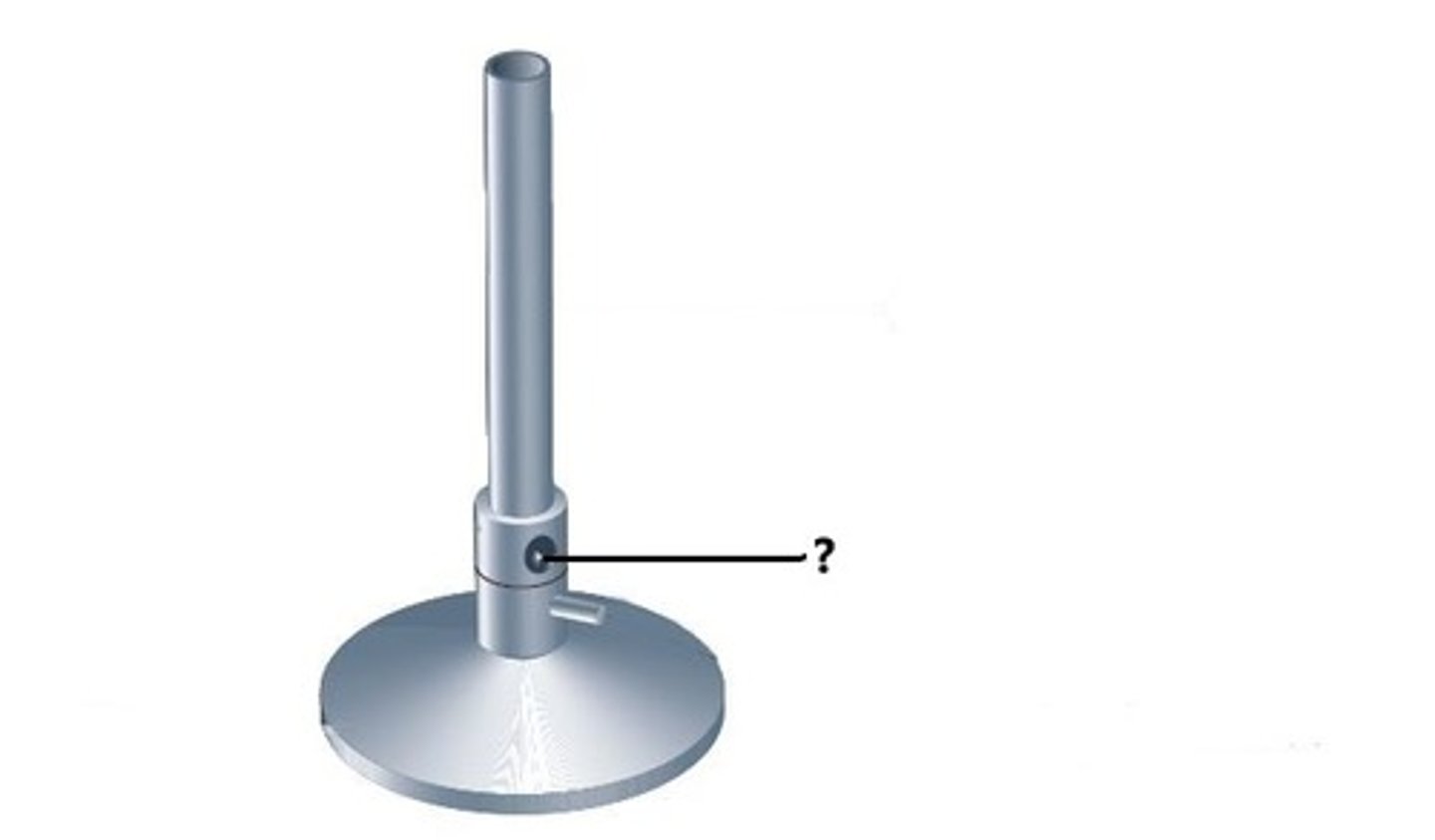
Collar
Controls the amount of air entering the barrel.

Base
It helps to provide support to the burner.

Gas valve
The gas receiving part. The gas supply to the burner can be controlled by rotating this left or right.

Complete combustion
A combustion reaction with a blue flame and an open airhole. There is enough oxygen to burn the fuel. Produces carbon dioxide.
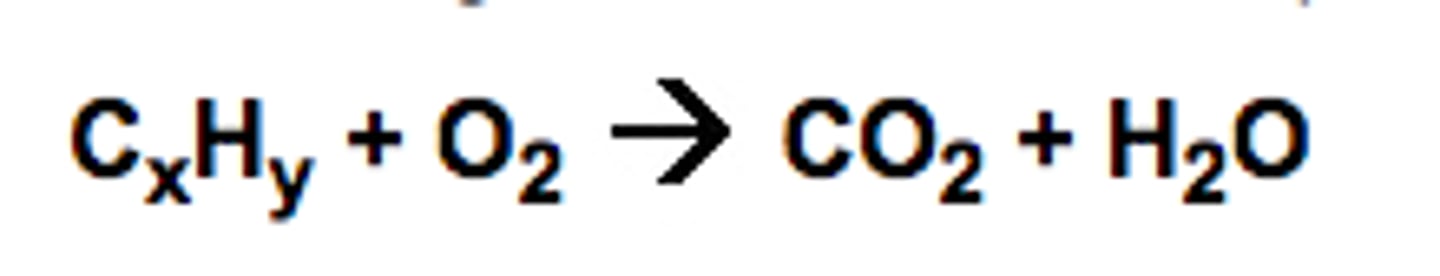
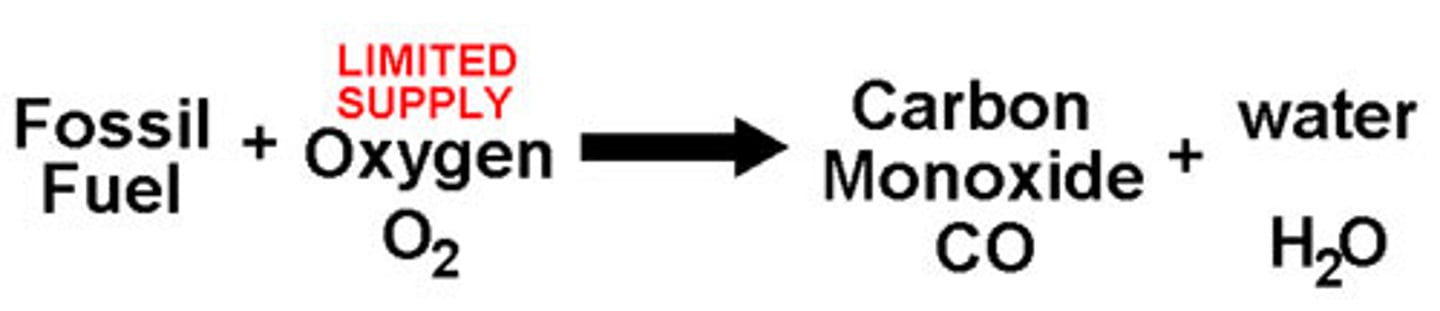
Incomplete combustion
A combustion reaction with a yellow flame and a closed airhole. There is not enough oxygen to burn the fuel. Produces carbon monoxide.