Exothermic and Endothermic reactions
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
15 Terms
What is an Exothermic reaction
An exothermic reaction is one that transfers energy to the surroundings so the temperature of the surroundings increases.
What are the three examples of Exothermic reactions
Combustion
Neutralisation reactions
Many oxidation reactions
What’s re the everyday uses of Exothermic reactions
-Hand warmers
-Self-heating cans
Draw a Exothermic energy profile
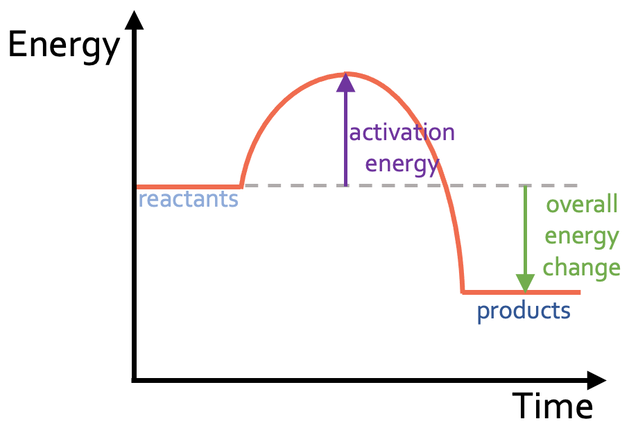
What is a Endothermic reaction
An endothermic reaction is one that takes in energy from the surroundings so the temperature of the surroundings decreases.
What are examples of Endothermic reactions
-Thermal decomposition
-Reaction of citric acid and sodium hydrogen carbonate
What is a every day uses of endothermic reaction
Sports injury sockets to all them to become instantly cooler.
Draw a endothermic energy profile
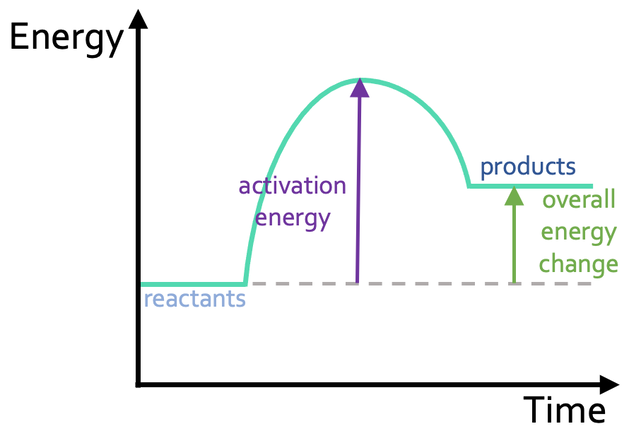
When can chemical reactions occur
Chemical reactions can occur only when reacting particles collide with each other and with sufficient energy
What is Activation energy
The minimum amount of energy that particles must have to react is called the activation energy.
When breaking bonds what process is required
Endothermic
When making bonds what process is required
Exothermic
In an Exothermic reaction energy released from forming new bonds….
Is greater than the energy needed to break existing bonds
In an endothermic reaction, the energy needed to break existing bonds is
greater than the energy released from forming new bonds
What the equation for finding overall energy change
Energy requires to break bonds(Left side) - energy released by forming bonds