Dry powder inhalers (DPIs)
1/41
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
42 Terms
What are DPIs?
These are inhalers where the drug inside them is a dry powder.
It is driven by the patients breathing rate, therefore they are automatically "breath-actuated".
Its carbon foot print is not based on its use, only on its manufacture.
What is the main problem associated with DPIs?
DPIs are controlled by the energy from a patient's breathing rate.
Therefore the patient can receive a variable dose depending on any changes in breathing rate.
This means there is variation with an individual patient's dose and across a population.
What is aerosolisation?
The process of converting a dry powder into an aerosol
Explain how DPIs produce an aerosol (aerosolisation)
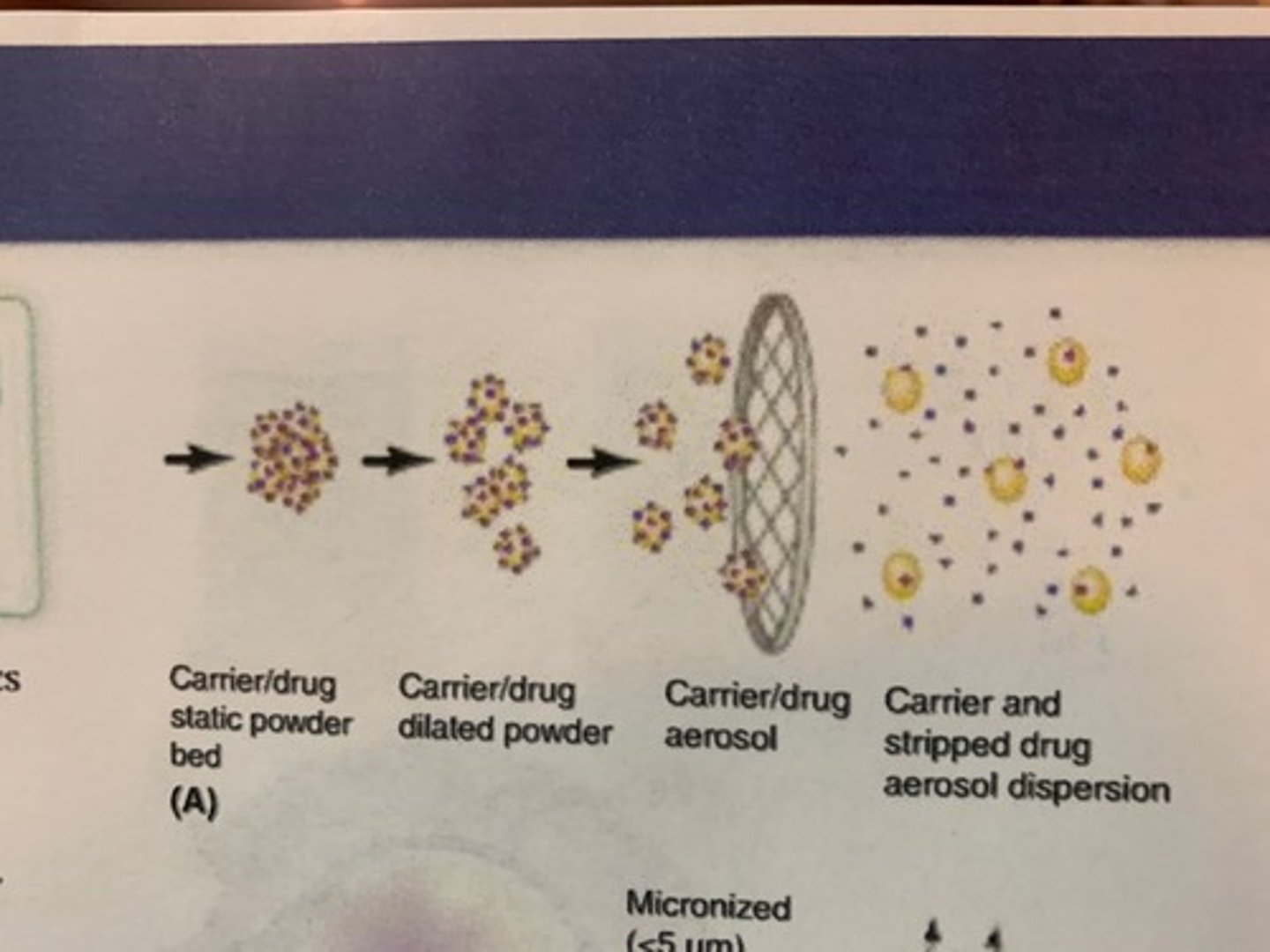
1. Fluidisation
- Static bed: At first particles aren't doing anything
- There is a pressure drop when patient begins to inhale.
- When the pressure difference between the top and bottom of the particles is large enough the particles become fluidised to produce a dilated powder bed
2. Entrainment
- Here particles begin to fly through the air out of the mouth piece of the inhaler.
- Particles are agglomerated (stuck together), so are too large to impact in alveoli or bronchioles.
3. De-aggregation
- Particles are pulled apart in airflow via various forces.
- Now, not all, but most particles are small enough to be inhaled into the lungs.
- Devices can mesh inside the mouthpiece which encourage de-aggregation.
Why is a patient's breathing rate so important for DPIs?
Patients need to generate a minimum inhalation flow rate (Q) for fluidisation and entrainment of the formulation and de-aggregation of drug for delivery into the lungs.
Therefore a variation of inhalation flow rate leads to variation of drug delivery.
Describe how de-aggregation of drug particles in a DPI occurs
Particles in the agglomerate are moving in the air and have many forces acting on them
1. Drag force
- Air is moving faster than the drug particles. There are large and small drug particles.
- Smaller particles want to move faster than the bigger particles so pull apart from the larger ones.
2. Centrifugal force
- Particle is spinning so particles pull apart.
3. Turbulences
- Air isn't flowing in the same direction.
- It is chaotically swirling around.
- Turbulence can also cause particles to pull away from one another.
- This can change the direction of the particles too and cause collision.
- Collisions cause further de-aggregation
4. Adhesion
- Sticking of particles together
What controls the forces causing de-aggregation?
Device and patient control:
- the airflow
Only the device controls:
- turbulence
Formulation can also affect likelihood of particles to collide with one another and the forces between the particles.
What is the fine particle fraction (FPF)?
The percentage of drug reaching the lungs.
For DPIs, this is usually around 30%.
What does the size of the fine particle fraction (FPF) depend on?
It depends on:
- Device design
- Patient inhalation
- Drug formulation
What is resistance?
How hard it is to breathe on an inhaler
What factors affect the delivery of drugs from DPIs?
1. Patient inhalation
2. Device design
3. Drug formulation
Describe the relationship between turbulence and resistance
The greater the turbulence the greater the resistance.
We don't particularly want resistance (side effect of the inhaler design) but it isn't too big of a problem for drug delivery.
Describe the importance of device design
Drug design is important because it can cause de-aggregation of particles via turbulence in airflow. The more de-aggregation, the more drug delivered to the lungs.
Turbulence: chaotic changes in airflow direction and velocity.
Different DPI devices have different levels of resistance e.g. pMDIs have no resistance as no need for turbulence.
Give examples of inhaler device designs to increase turbulence
1. Ellipta
- Airflow is split into two. The two airstreams mix to give turbulence.
2. Turbohaler
- Air flow is again split into two channels as well. Air then it twists and spins around which gives turbulence.
Describe the relationship between device and airflow
The higher the resistance, the harder the it is to breathe through it. This is an issue with elderly people as for a high resistance device need to breathe fast enough.
The high air resistance isn't all bad though because we can use a lower flow rate.
.Higher resistance = lower flow rate for same amount of patient effort
What are some benefits of higher resistance devices?
1. Higher resistance devices generally perform well at lower flow rates
- most patients can use high resistance devices
2. High resistance devices can reduce flow rate variation and oropharyngeal deposition
Describe how patient inhalation affects the delivery of drugs from DPIs
Speed of inhalation causes dose variation.
We want patient to achieve a peak flow rate quickly so that drug particle de-aggregation occurs quicker.
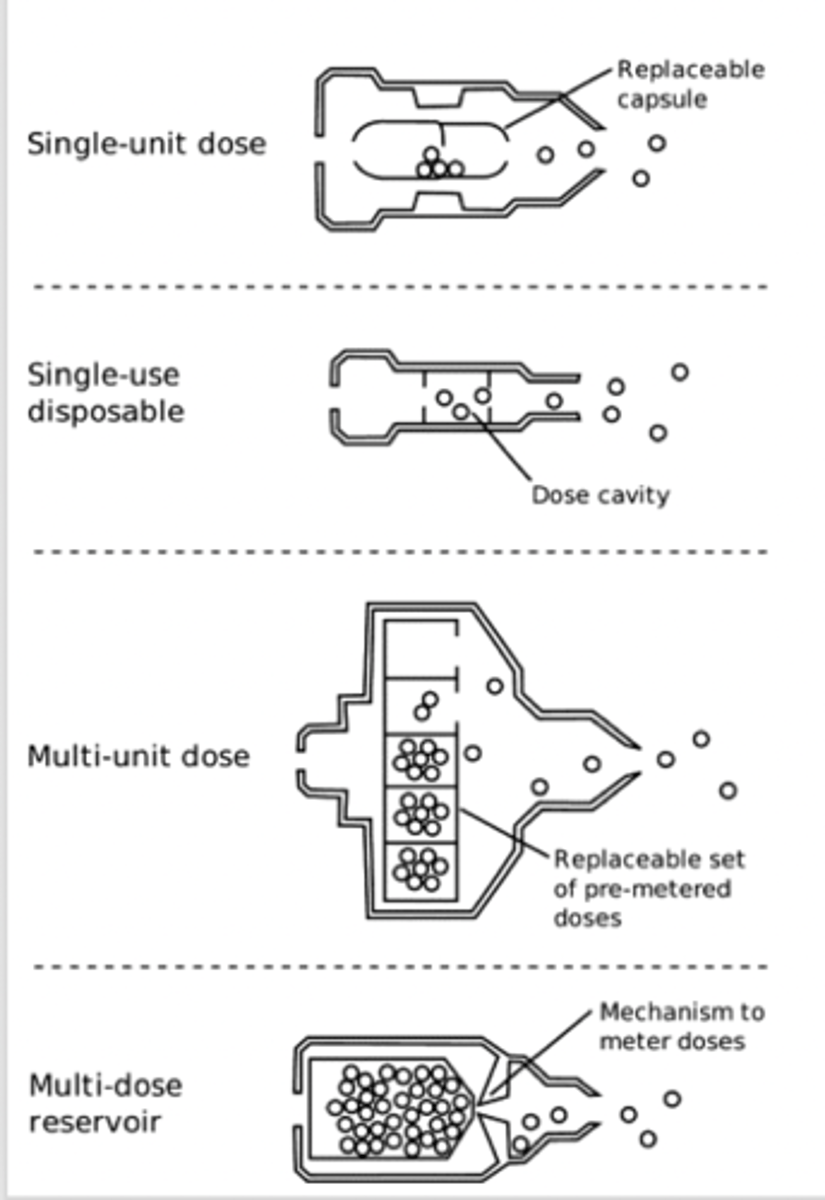
What are the different types of DPI device?
1. Single-unit dose (disposable)
2. Single-unit dose (reusable): device reusable. Usually have dry powder as a capsule. May be difficult for patients to use
3. Multi-unit dose: dose measured in factory. Put into a foil blister. Either reusable blisters multi-unit disposable or more modern type where the blisters are inside them
4. Multi-unit dose reservoir: There is a way to measure the dose and protect the powder (from moisture).
What is the size requirement of particles in DPI formulations?
The drug particles must be small enough to reach the lungs (3-5 micrometres).
What controls the behaviour of particles in a DPI?
The behaviour of particles for DPI formulations (3-5 micrometres) is dependent on their adhesion to other particles and surfaces.
This is because the smaller the particle, the lower the effect that gravity has on particle behaviour.
What factors affect the formulation (drug particles behaviour) of DPI powders?
Particle interactions in a DPI are mainly controlled by these main adhesive forces:
1. Van der Waals forces
2. Capillary forces
3. Electrostatic forces
Describe how Van der Waals forces affects the formulation (drug particles behaviour) of DPI powders
Van der Waal forces when two opposing temporary dipoles are next to one another.
Temporary dipoles form when the cloud of electrons dominate on one side to create a temporary negative charge and there is a deficit of electrons on the other side creating a positive charge.
This only occurs over small distances.
This is a force universal for solids
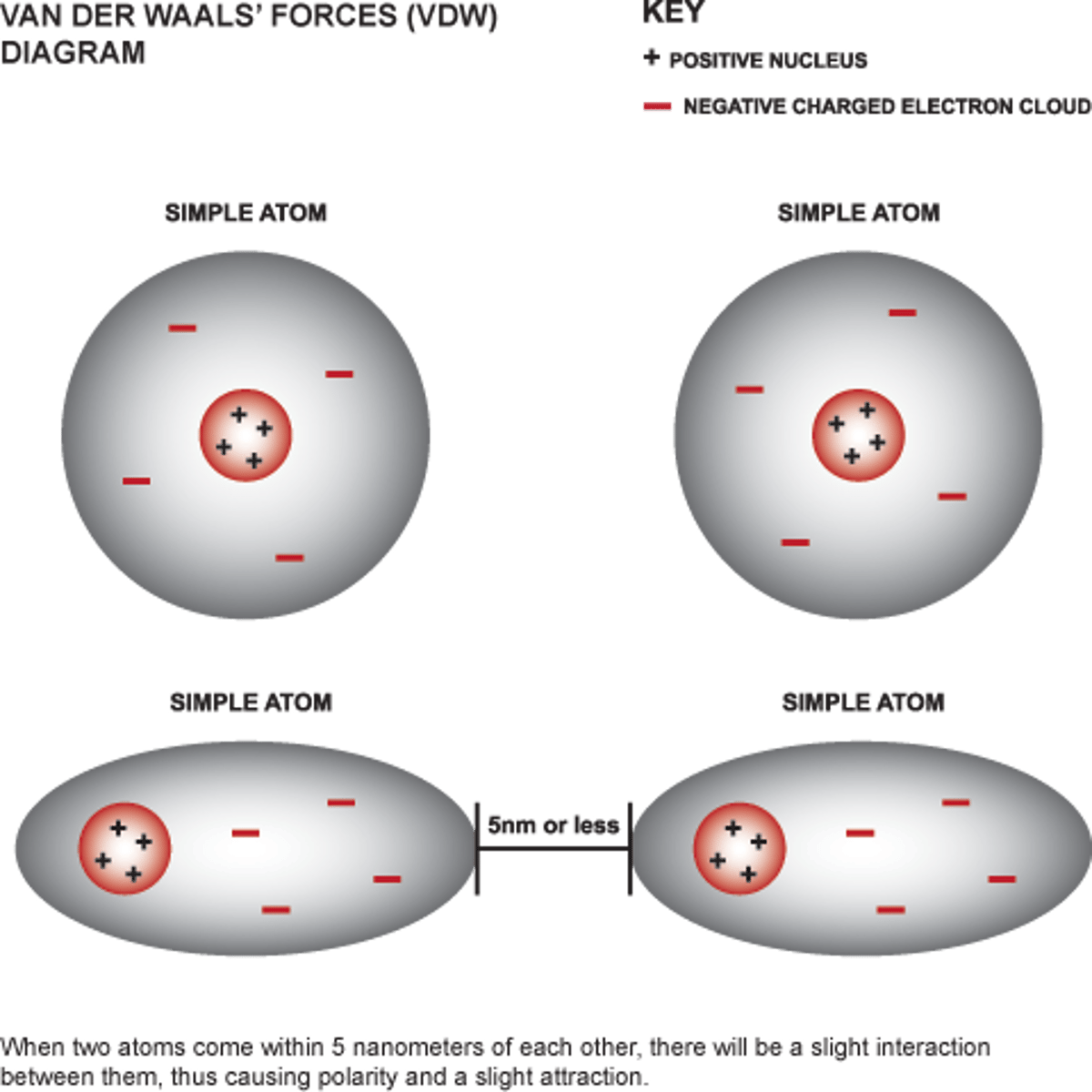
When do Van der Waals forces dominate?
- Low humidity
- Absence of electrostatic forces
Describe how capillary forces affects the formulation (drug particles behaviour) of DPI powders
Capillary forces is when there is condensation of water vapour between contiguous bodies to form a liquid bridge.
The strength of the force depends on the hydrophobicity of the surface and the water in the atmosphere.
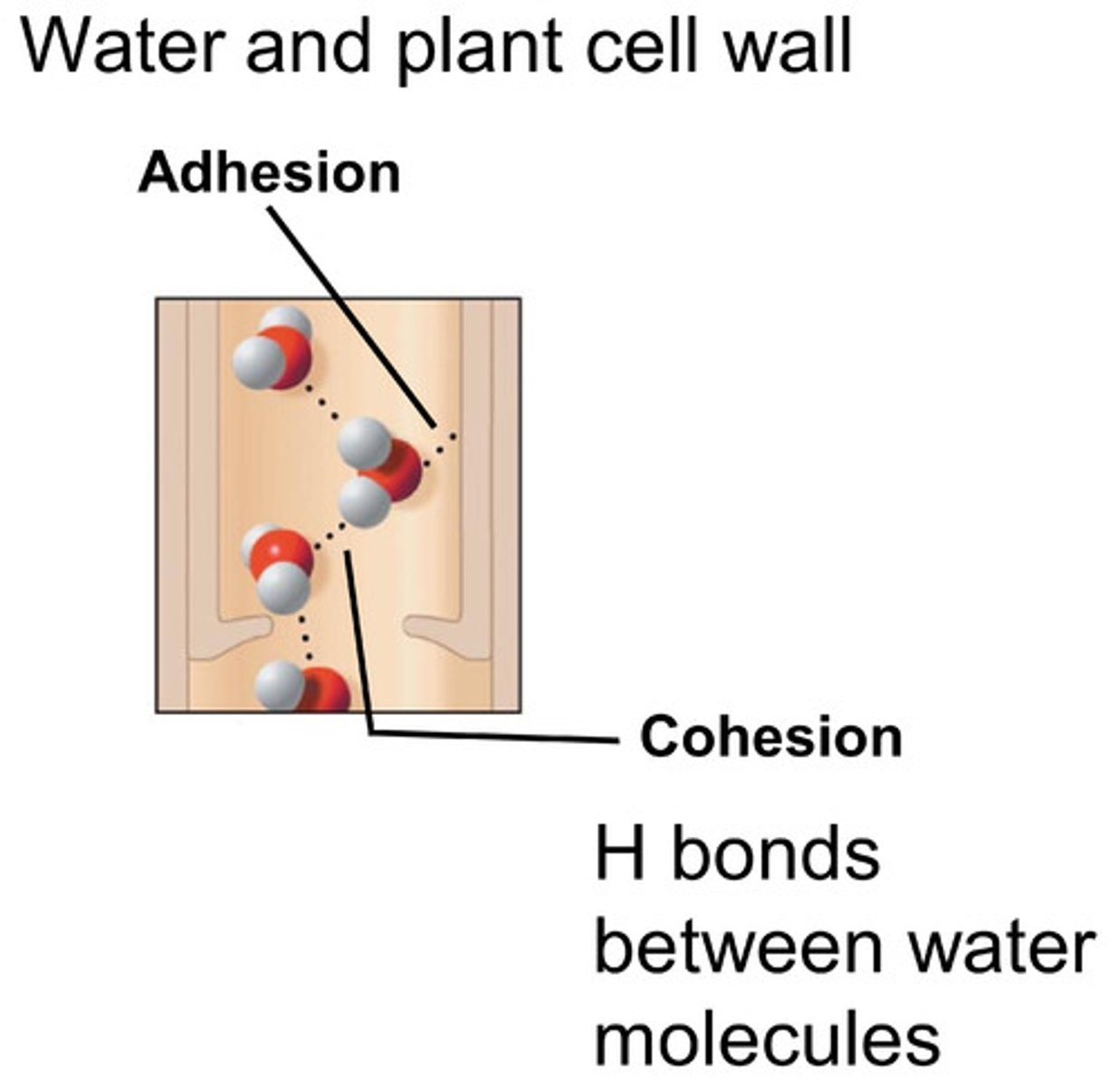
When do capillary forces dominate?
When humidity is >50-60%
Describe how electrostatic forces affects the formulation (drug particles behaviour) of DPI powders
Electrostatic forces are caused by frictional contact between dissimilar materials.
This force can either be attractive or repulsive and occur at long distances.
When do electrostatic forces dominate?
At low humidity
Why is it difficult to produce dry powder formulations for DPIs?
Our particles are dominated by adhesion.
This makes producing an inhaler harder as powders made of small particles don't want to flow.
They prefer to stick to things (due to adhesion forces).
For DPIs, we want to make powder formulations which flows and have small drug particles (3-5 micrometres)
Why is good flow important for a DPI formulation?
1. To manufacture the inhaler
2. To help fluidisation
What factors can be manipulated to alter adhesive forces formed in DPI formulations?
1. Particle size
2. Particle shape
3. Surface roughness
4. Surface chemistry (i.e. micronized particles)
5. Humidity
These are changed to optimise flow and aerosolisation properties. These properties must be kept constant during manufacture and the shelf-life of the drug.
Describe how drug formulation affects the delivery of drugs from DPIs
1. Drug - Drug Interactions (Cohesion)
2. Drug - Excipient Interactions (Adhesion)
3. Drug - Device Interactions (Segregation)
The balance of these things will affect adhesion and how the drug impacts in the lungs
What strategies can we use to control drug-drug, drug-excipient, and drug-device interactions?
1. Carrier based systems: a mixture of small drug particles with larger excipient particles.
2. Agglomerated-systems
Describe carrier-based systems
Adhesive bond formation
This is the most common formulation.
This is when we blend drugs with a carrier.
The properties which control FPF (fine partial fraction) in carrier-based systems:
- Particle size distribution
- Particle shape: we can add bumps to the particles - this makes the drug more distant, fewer van der Waals (form close bonds).
- Surface roughness: less rough increases FPF
- Carrier material
- What polymorph of the drug crystal forms
An example of this system is microionised drug with carrier lactose.
What are advantages of a carrier-based formulation for DPIs
1.Allows accurate metering of small quantities of potent drug
2. Flows easier so easier handling and processing
What is fine particle lactose blending (FPL)?
Fine particle lactose (fines) sticks to the sticky areas so drug goes to less sticky area.
Addition FPL to DPIs improves FPF (fine particle fraction).
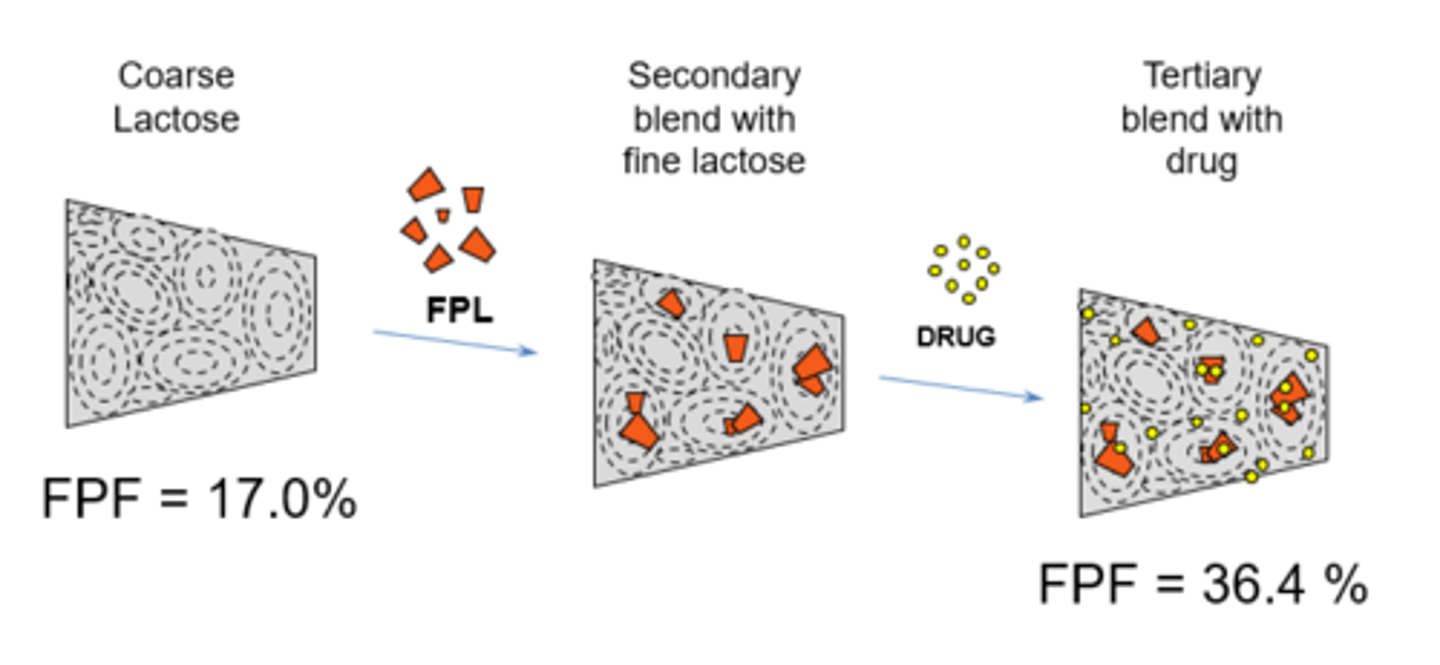
Describe agglomerated powder systems
This is when for high dose drugs where carrier-based systems are not feasible.
1. Free flowing" macroscopic agglomerates can be produced via cohesive bond formation = cohesive agglomerate formed (high energy and high free surface area).
2. The drug-containing particles are separated in the airways due to the turbulent airstream.
It is necessary for the agglomerates to de-aggregate in order to be small particles inhaled in the lungs.
What is the likely future of drug formulation for DPIs?
Engineered particles.
For example adding bumps to the small drug particles particles to prevent them sticking to one another. Therefore, we make the drug more distant, so fewer van der Waals forces form (Van der Waals form close bonds). Therefore we can do this to our dry powder formulation.
Explain the advantages of DPIs
1. Naturally breath actuated - patient can deliver at any time, no need for hand-breath coordination.
2. Stability is more likely because it is in a solid and dry form
3. Propellant free - more environmentally friendly
4. Some have no excipients
5. Can deliver relatively large doses
Explain the disadvantages of DPIs
1. De-aggregation depends on patient's ability to inhale
2. To use and breathe particles apart we ned to breathe quickly. Also, increase inhaled air velocity, increased de-aggregation of particles but increased potential for inertial impaction.
3. Increased humidity can decrease stability
4. Less efficient at getting drug in to the lungs compared to pMDI (if pMDI is used perfectly).
5. Patient may not be able to set up inhaler if multi-dose disposable - e.g. patients with arthritis - Need to make sure patient breathes properly AND can set the inhaler up to use.
Explain inhaler technique for DPIs
1. Load dose - different for each device
2. Breathe out as far as possible away from the inhaler.
3. Place inhaler in mouth and seal lips around the mouthpiece
4. Breathe in QUICKLY and deeply for energy to de-aggregate the particles.
4. Accelerate breathe as quickly as possible (need to increase energy quickly as initial flow rate is important)
5. Hold breath for around 10 seconds
What devices can be used to help practice inhaler technique with DPIs?
We can use:
1. In-check dial
2. Vitalograph AIM
to aid patients to using DPI inhalers
What is the main difference in inhaler technique between pMDIs and DPIs?
Speed of breath is the main difference between pMDI and DPI
For pMDIs we inhale SLOWLY while simultaneously pressing the canister
whereas for DPIs we:
breathe in quick and deep.
Therefore it is better to give a patient one type of inhaler because they can become confused by different inhaler techniques.