SECTION 02: CHEMICAL REACTIONS AND ENZYMES AS CATALYSTS
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
🧪 What is ΔG?
ΔG = Gibbs Free Energy
Tells us if a reaction can happen on its own (spontaneous)
Formula:
👉 ΔG = ΔH – TΔS
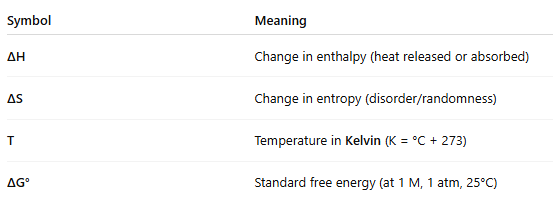
📘 What do the symbols mean?
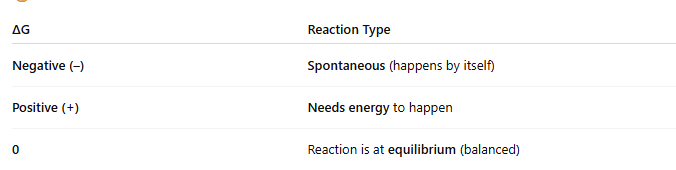
🧭 What does ΔG tell us?
💡 Study Tips:
Free energy = energy available to do work
Enthalpy = heat change
Entropy = disorder
Gibbs Free Energy brings all 3 together to predict if a reaction can happen
✨ Summary:
ΔG helps predict whether a reaction is spontaneous, non-spontaneous, or at equilibrium, based on changes in heat (ΔH), disorder (ΔS), and temperature (T).
🧪 What is ΔG (Gibbs Free Energy)?

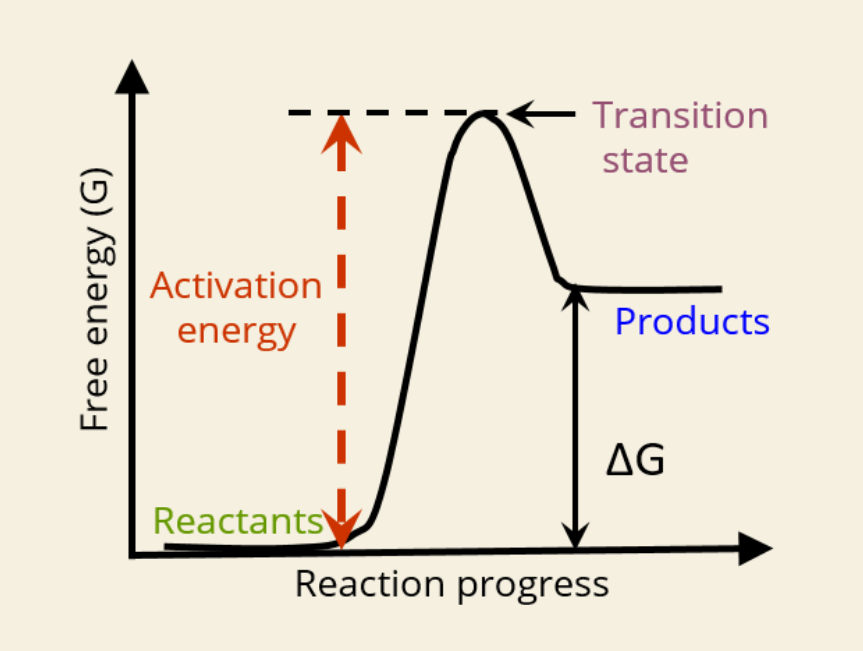
🚀 What is Activation Energy?
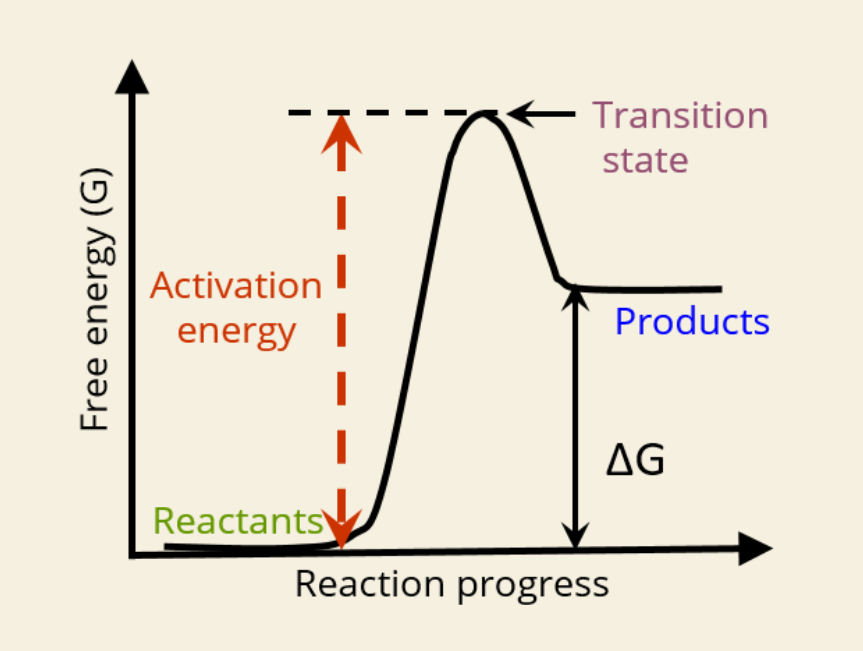
The initial energy needed to start a reaction
Required to reach the transition state
🔺 Shown as the height from reactants to the peak in the graph
🔄 What is the Transition State?
A high-energy unstable state between reactants and products
Reaction must pass through this point
First Graph (Exergonic Reaction)
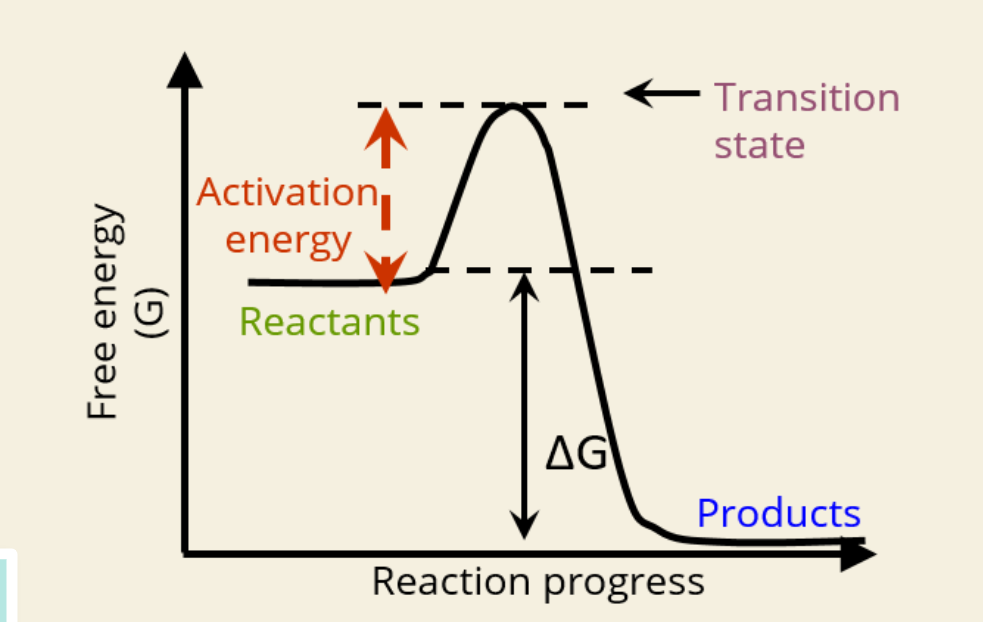
Reactants have more energy than products
ΔG is negative → spontaneous
Activation energy still needed to start
Releases energy to surroundings
Second Graph (Endergonic Reaction)
Products have more energy than reactants
ΔG is positive → not spontaneous
Needs continuous energy input
Often coupled with exergonic reactions in cells
🧠 BONUS TIPS:
Exergonic = energy exits system → spontaneous
Endergonic = energy enters system → non-spontaneous
Don't confuse with exothermic/endothermic, which refer to heat (ΔH), not free energy
✨ Summary:
ΔG shows whether a reaction happens on its own. Exergonic = spontaneous, Endergonic = needs help. Reactions still require activation energy to reach the transition state, even if spontaneous.
🔥 What is a catalyst?
A substance that speeds up a reaction
It lowers the activation energy needed to start the reaction
❗ It does not change ΔG (reaction spontaneity stays the same)
🧪 What do enzymes do?
Enzymes are biological catalysts
They lower activation energy by:
Stabilizing the transition state
Using weak interactions at the active site
📊 What does the image show?
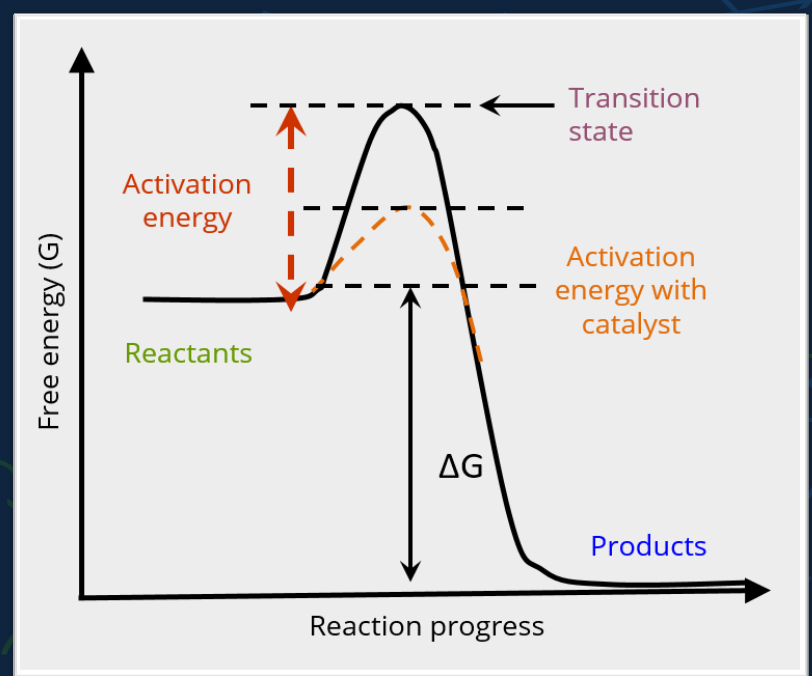
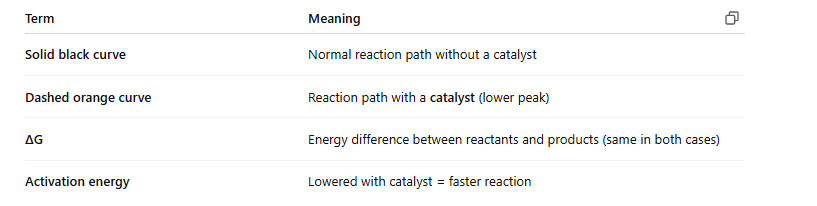
✅ Key Points:
Catalysts speed up reactions, but don’t change their final outcome
Enzymes work by making it easier to reach the transition state
ΔG stays the same with or without a catalyst
✨ Summary:
Catalysts (like enzymes) make reactions faster by lowering activation energy, not by changing the spontaneity (ΔG). This allows reactions to happen more easily, especially in biological systems.
🧪 Key Terms:
E = Enzyme
S = Substrate
ES = Enzyme-Substrate Complex
EP = Enzyme-Product Complex
P = Product
k₁, k₋₁, k₂ = Rate constants (speed of forward/reverse steps)
🔢 Step-by-Step (Image Numbered 1–4):
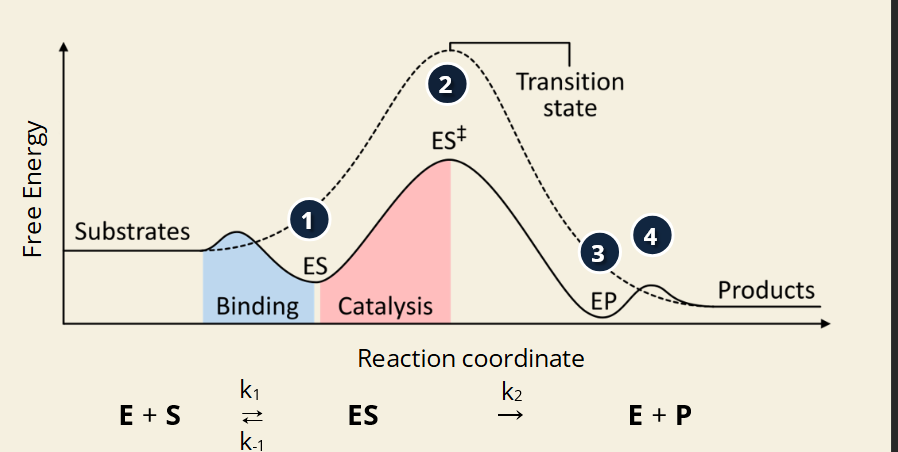
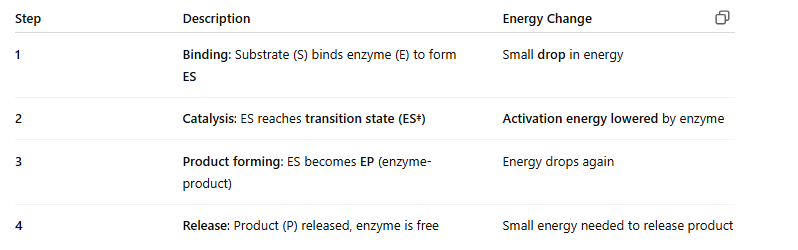
📊 Image Highlights:
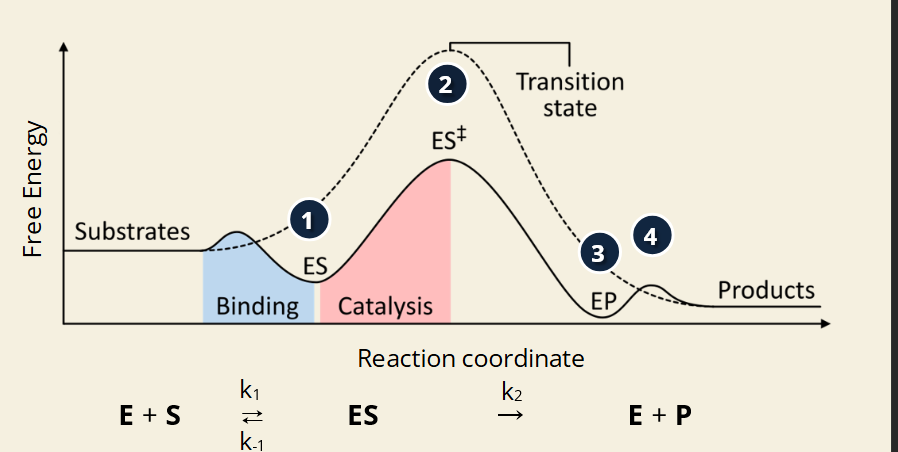
Solid curve = enzyme-catalyzed reaction
Dotted curve = uncatalyzed (no enzyme) = higher peak = more energy needed
Transition state (ES‡) is the highest point = energy "hill"
Enzyme lowers the height of the hill = faster, easier reaction
🔑 Important Notes:
Enzymes don’t change ΔG (overall energy difference)
They only lower activation energy
Each rate constant (k₁, k₋₁, k₂) controls how fast each step happens
✨ Summary:
Enzymes make reactions faster by lowering activation energy. The reaction has multiple steps: binding, catalysis, product formation, and release. Enzymes do not change the final energy output (ΔG), just how easy it is to get there.
⚡ Why do reactions need energy?
Even spontaneous (exergonic) reactions need an initial push of energy → this is called activation energy (Ea)
On early Earth, energy came from lightning, heat, sunlight
Today, we get energy from food, but it must be converted into something usable—like ATP
⚙ What is ATP?
ATP = energy carrier in cells
When ATP is broken, it releases energy that fuels reactions in the body
📈 What is the transition state?
A temporary high-energy point during a reaction
Old bonds are breaking, new ones are forming
It only lasts a fraction of a second
🚧 Why are reactions slow?
The activation energy acts like a barrier
Without help, reactions happen too slowly to support life
🧪 What do catalysts (like enzymes) do?
Lower activation energy
Speed up reactions without being used up
Make reactions happen faster and easier
🧬 How do enzymes work?
Enzymes = biological catalysts
Each enzyme has an active site shaped for its specific substrate
When the enzyme binds the substrate:
It brings atoms close together
Stabilizes the transition state
Helps break old bonds and form new ones
Product is released → enzyme is reused
🔑 Lock & Key Model:
Enzyme = Lock
Substrate = Key
Only the right substrate fits perfectly
🌀 Induced Fit Model:
The enzyme slightly changes shape when the substrate binds
This tightens the fit and stretches the substrate’s bonds
Makes it easier to break and form bonds (lowering activation energy even more)
✅ Summary:
Enzymes speed up reactions by lowering activation energy. They bind specific substrates at the active site, stabilize the transition state, and help the reaction happen faster. Two models explain this: Lock & Key (perfect fit) and Induced Fit (flexible fit).
❓ What is a catalyst?
catalyst - 1. Increase rates of reaching chemical equilibrium
catalyst - 2. Selectively increase certain products
✔ True
Catalysts (especially enzymes) can be very specific
They can favor certain reaction pathways, making more of a desired product
catalyst - 3. Decompose undesirable products
✔ True
Catalysts can help break down harmful or unwanted compounds
Example: Catalase breaks down hydrogen peroxide (a toxic by-product in cells) into water and oxygen
✨ Summary:
Catalysts are powerful tools—they speed up reactions, can favor useful products, and even help break down unwanted substances. That’s why the correct answer is "All of the above."