BIOC 202 - ATP Synthase & mitochondrial transport
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
27 Terms
what is the pH differential across the mitochondrial membrane?
1.4 pH units
what makes up the ATP synthase?
F₀ & F₁

what where is F₀ embedded, and what does it contain?
embedded in the inner mitochondrial membrane and contains the half channels that the protons flow thru. It uses this proton flow to generate ATP.
what does F₁ contain?
contains 3 alpha-beta subunits arranged in a ball (ATP synthesis occurs in Beta). In the center of this ball is the gamma (subunit gamma)
which subunit in F₁ spins?
subunit gamma spins. The alpha-beta subunit does not
the gamma shaft binds to each alpha-beta subunits differently, what does this cause?
different confirmations in each of the 3 alpha-beta subunits
what are the different conformations of the alpha-beta subunits?
alpha-beta loose site (L; B-ADP) - Loading conformation. ADP + Pi combines and become trapped
alpha-beta tight site (T; B-ATP) - ATP synthesis step. ATP is made and is tightly bound to beta-subunit
alpha-beta open site (O; B-empty) - release conformation. It has low affinity for ATP and ADP
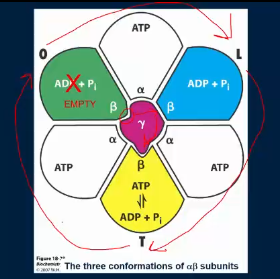
what does the gamma shaft spinning cause?
causes each of the 3 alpha-beta subunits to cycle thru the loose, tight, and open conformations which results in synthesis and release of ATP
how many H+ are needed to move into the matrix per ATP made?
4
3 to spin F₀
1 H+ to move Pi
how many ATP are generated per FADH2 mlc?
1.5
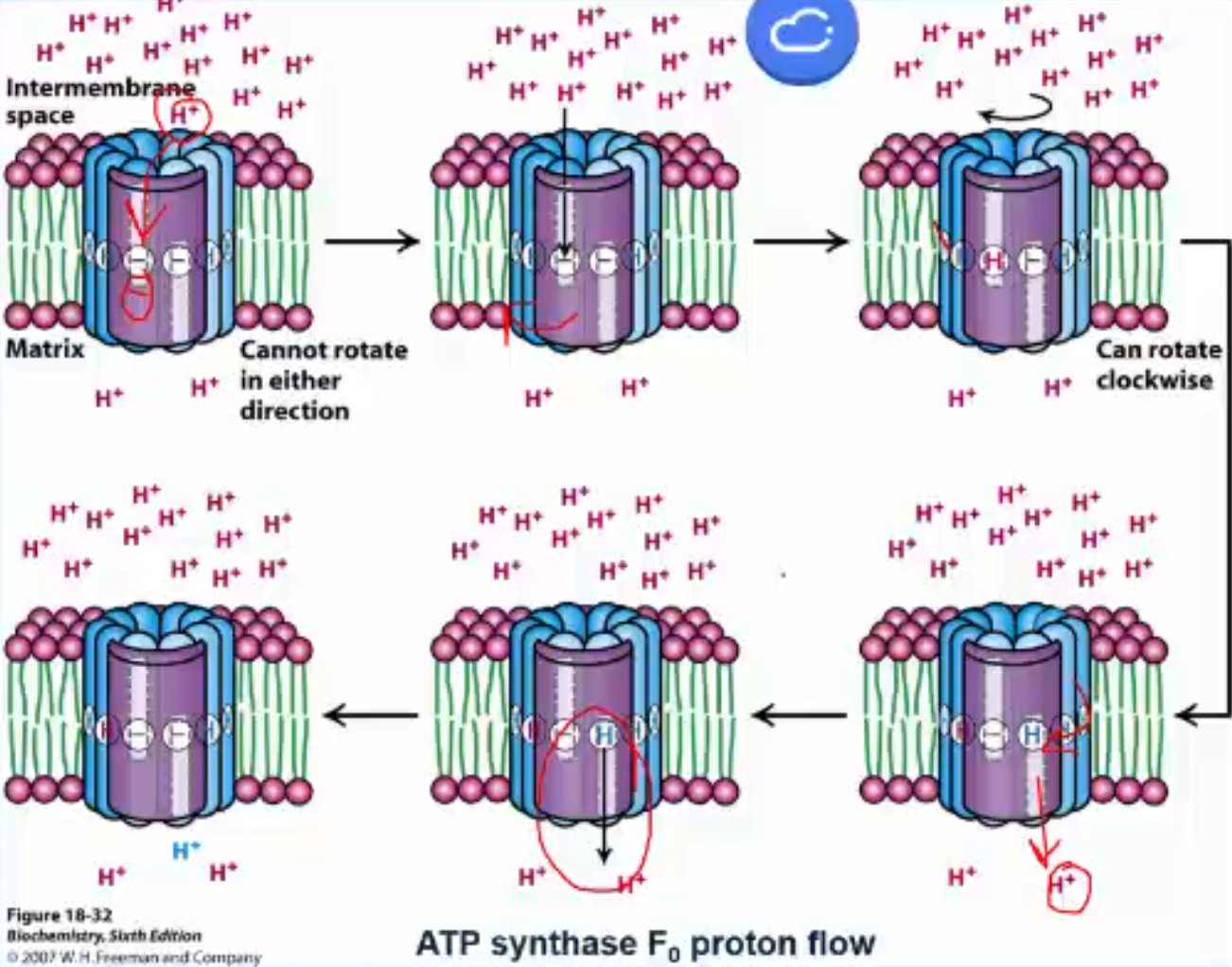
How does F₀ cause the gamma shaft to spin?
F₀ is composed of many subunits; we only focus on subunit c and a. Subunit c consists of 2 alpha-helices that span the membrane. There are 10-12 (10 in humans) c subunits arranged into a cylinder; this entire cylinder rotates. Halfway down one of the helices is a key aspartic acid/aspartate residue, which can be protonated or deprotonated depending on pH. The subunit a (aka the clamp) covers 2 subunits. It is stationary and has 2 half channels (one open to the IMS and one open to the matrix). Now a subunit c can only move into the subunit a clamp when charged (clamp masks the charge from bilayer)
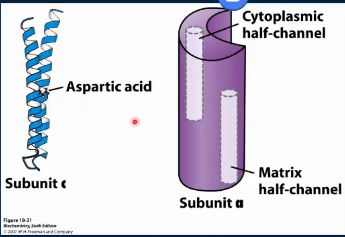
How does F₀ use the H+ to spin?
a charged aspartate subunit c is in the IMS half chain, a uncharged aspartic acid is in the matrix half chain.
a H+ diffuses from the IMS, thru the IMS half channel to protonate the aspartate to aspartic acid and now this subunit is uncharged
The entire subunit complex (cylinder) can rotate clockwise by one subunit c. The freshly protonated uncharged subunit c moves into the membrane.'
This brings a charged subunit c into the IMS half channel and an uncharged subunit c into the matrix half channel
a proton diffuses off the aspartic acid, down the matrix half channel and into the matrix. This subunit c is now uncharged and the cycle repeats.
Note: a proton from the IMS binds a charged subunit c and then goes almost one full rotation of the cylinder before being released into the matrix
what happens when [ATP]/[ADP][Pi] ratio is high?
there is little ADP to be phosphorylated and O2 consumption drops (normally, ATP synthesis and the ETC are coupled)
when [ATP]/[ADP][Pi] ratio is low, reverse is known as ______. What is ____?
acceptor control
acceptor control is the regulation of cellular respiration by the availability of ADP as a phosphate receptor
in what situation can ATP synthase not spin?
if ADP and Pi are not bound to the loose alpha-beta subunit
what do uncouplers do? what whare the diff types of uncouplers?
Uncouplers transport protons w/ out specific channels and ruin proton gradient
chemical uncouplers → DNP + FCCP
uncoupling protein 1 (UCP1) → uncoupling
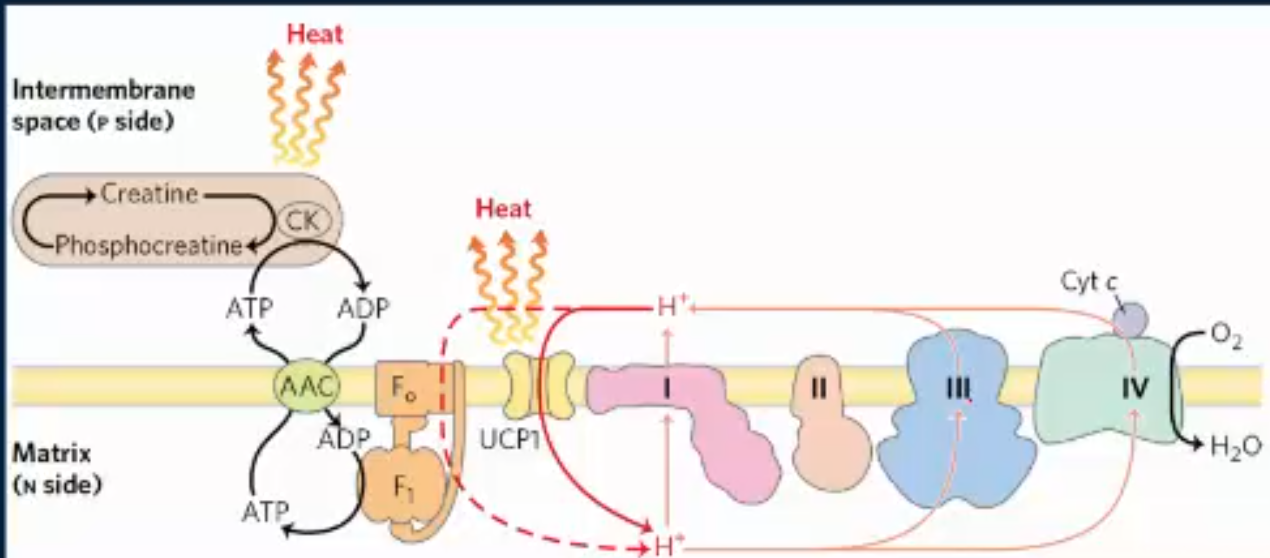
what is an example of a mlc that can uncouple the ETC and ATP synthase? how does it do this?
2,4-dinitrophenol can uncouple the ETC and ATP synthase by carrying H+ across the IMM. This reduces the H+ gradient and the ETC speeds up to restore it (burning more glucose), but the rate of ATP synthase stays the same (or if 2, 4-DNP is rlly high, it drops) and heat is produced.
what does “brown fat” do?
carries thermogenin (aka uncoupling protein 1), and this is found in newborns and mammals adapted to the cold. This is going to uncouple the IMM, generating heat as a result. In other words, thermogenin allows mammals to stay warm in cold environments since it converts potential energy from the proton motive force into heat
how do e- on the NADH in the cytoplasm get to the ETC?
by shuttles
what are the shuttles that transport electrons to the ETC? where are they located?
glycerol-3-phosphase shuttle (skeletal muscle, brain)
malate-aspartate shuttle (liver, kidney, heart)
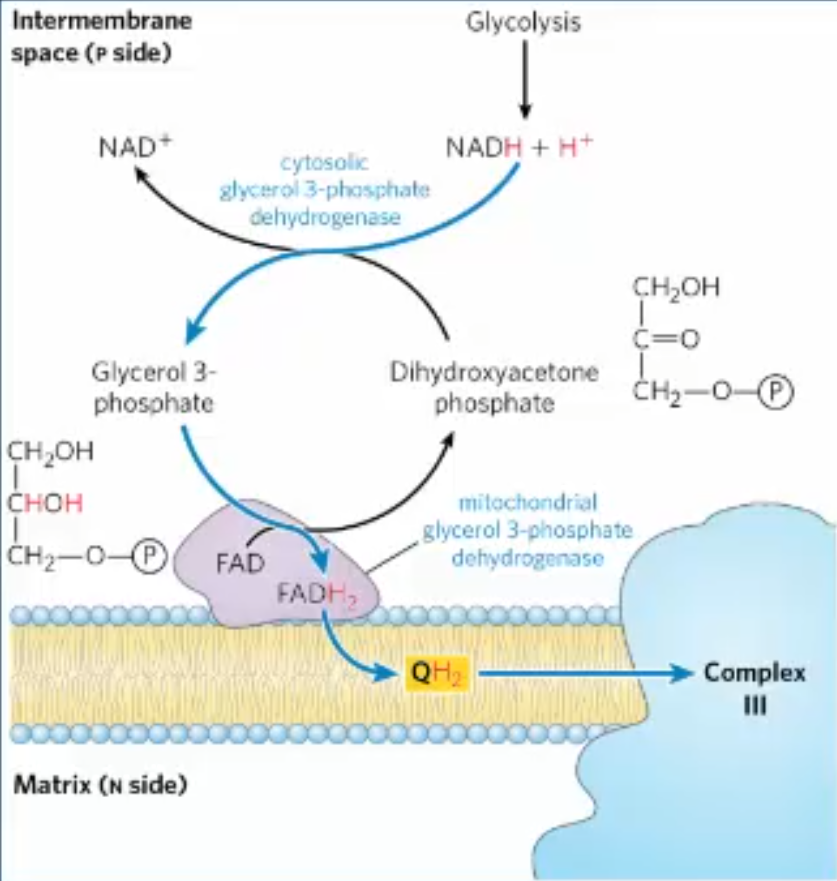
how does the glycerol-3-phosphate shuttle work?
cytosolic glycerol-3-phosphate dehydrogenase reduces DHAP to glycerol-3-phosphate, oxidizes cytosolic NADH to NAD+. Glycerol-3-phosphate is carrying 2e- from NADH
IMM bound glycerol-3-phosphate dehydrogenase oxidizes glycerol-3-phosphate back to DHAP, in the process, transferring 2e- to FAD, forming FADH2
FADH2 passes the 2e- to Q, reducing it to QH2
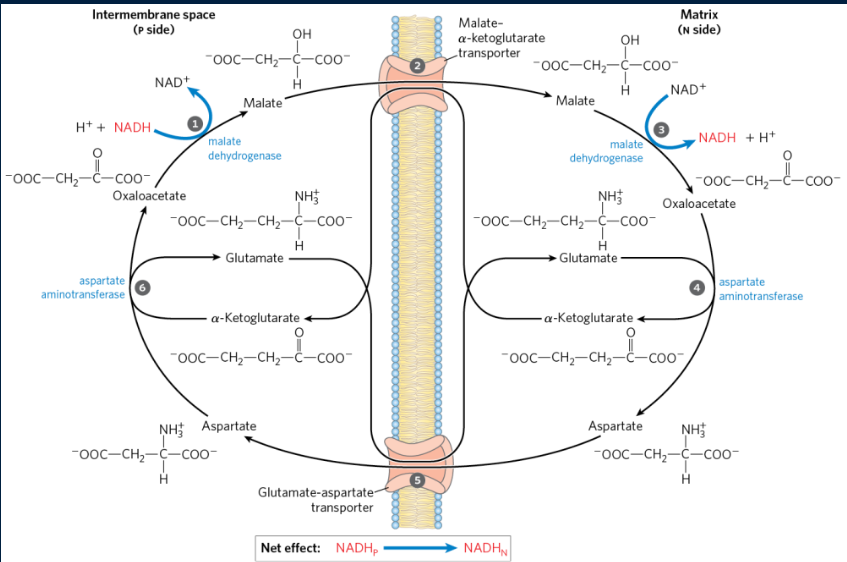
how does the malate-aspartate shuttle work? (explain + draw)
oxaloacetate in the cytosol is reduced by NADH to malate. This is done by cytosolic malate dehydrogenase
malate (carrying the 2e-) is transported into the matrix by the malate I alpha-ketoglutarate translocase
malate is oxidized back to oxaloacetate by mitochondrial malate dehydrogenase, generating NADH in the matrix. NADH can go thru complex I, but we have a problem - oxaloacetate cannot be transported back to the cytosol as there is no oxaloacetate translocase.
oxaloacetate is transaminated by glutamate, forming aspartate and alpha-ketoglutarate in the matrix
the glutamate/aspartate translocase moves aspartate into the cytosol. alpha-ketoglutarate is also moved into the cytosol by malate alpha-ketoglutarate translocase
in the reverse of 4, aspartate transaminates alpha-ketoglutarate, reforming oxaloacetate
how are ATP and ADP transported?
by the ATP/ADP translocase. ATP in the matrix (goes to cytosol) in exchange for 1 ADP from the cytosol (goes to matrix)
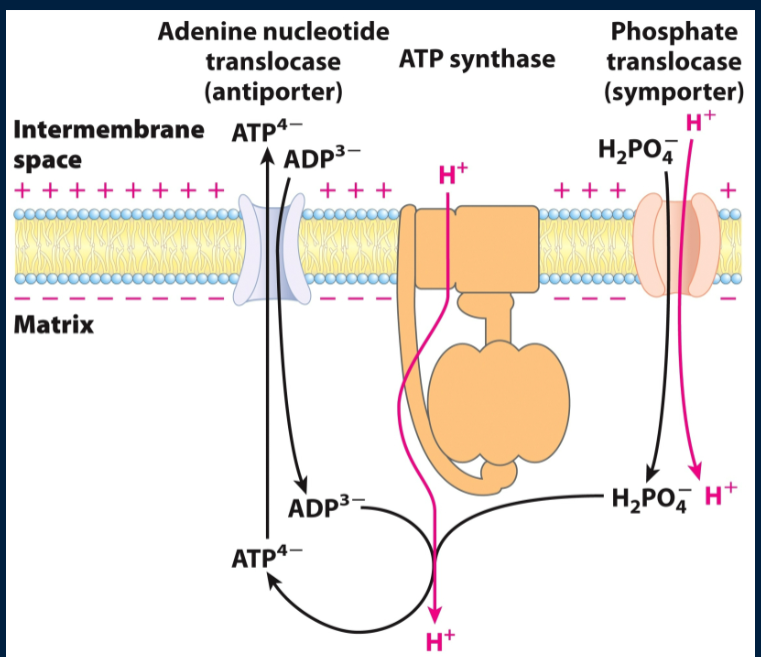
what is ATP/ADP transportation driven by?
the charge gradient created by the proton motive force. ATP has a charge of -4 and ADP has a charge of -3 and the IMS is positively charged. ATP more neg charged than ADP so will wanna go to the IMS
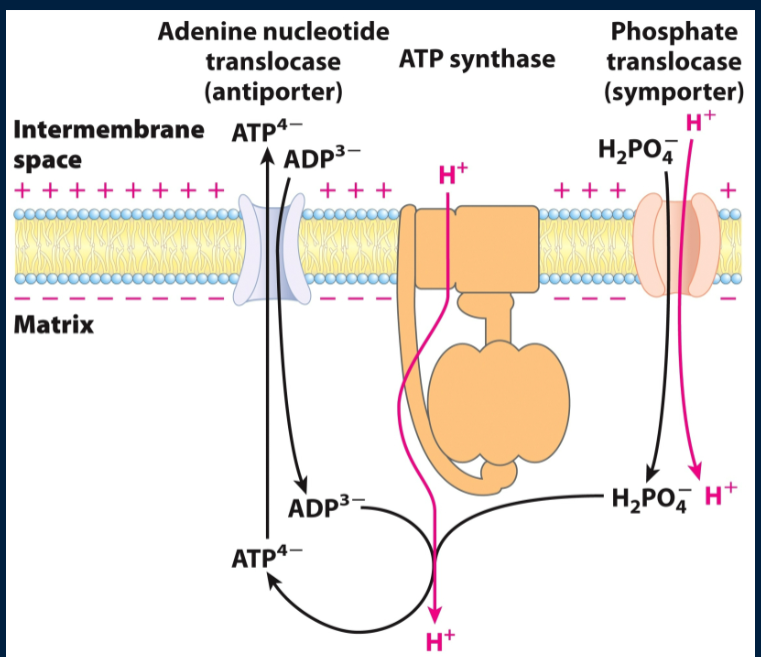
what other translocase besides the ATP/ADP translocase is needed for ATP/ADP transport? How does it work and what is it driven by?
phosphate translocase transports Pi to matrix (so that it can go with ADP. To do this, it needs to pump 1 H+ with the phosphate intro the matrix (thus sacrificing a proton from the cytosol). Driven by PMF
what is pyruvate transported into the matrix by?
pyruvate translocase
make a table of pathway, e- carriers, and ATP. Pathways: glycolysis, PDC (2 cycles), Krebs cycle (2 turns), oxidative phosphorylation, NADH from glycolysis, NADH from PDC, NADH from Krebs, FADH2 from Krebs
look at lecture 31 notes for answers