Chapter 5 BSC-2010 Biomolecules
1/88
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
89 Terms
Be able to list the four major classes of biomolecules and mention which is not a macromolecule and why.
carbohydrates
proteins
nucleic acid
lipids (not polymer or macromolecules)(Lipids are not polymers, because they are not built from monomers and are not covalently bonded.
Be able to distinguish
-monomers and
-polymers based on their given definition.
Large polymers are known as macromolecules
-is a long molecule consisting of many similar building blocks linked by covalent bonds
-the repeating unit that serve as building blocks
-polymers are built from monomers
Be able to name the two fundamental chemical reactions that are used to make biopolymers and break biopolymers.
dehydration synthesis and hydrolysis
Be able to differentiate between dehydration reaction and hydrolysis reactions in living organisms based on the following criteria:
a. What do they accomplish (make polymer or break polymer)?b. Do they produce water or do they consume water? c. Do they need enzymes?
dehydration synthesis: occurs when 2 monomers bond together through the loss of a water molecule hydrolysis: disassembles polymers to monomers. reverse of dehydration synthesis.(realese a water molecule)
enzymes can speed up these chemical reactions
State the known functions of carbohydrates in living cells.
-include sugar and polymers of sugars
-simplest form are monosccahrides or simple sugars
-polysaccarides, polymers composed of many sugar building blocks
Short term energy storage and can help build structures such as exoskeletons for crabs through chitin and carbonitrate and cell walls made of cellulose
Be able to distinguish the terms monosaccharide, disaccharide and polysaccharide
-monoscaaharide have molecular formulas that are multiples of CH20 (glucose is an example)
-disaccharide is formed when a dehydration reaction joins 2 monosaccharides
this covalent bond between 2 monosaccarides is called glycosidic linkage
-polysaccharides: few hundred to a few thousand monosaccarides joined by glysodic linkage (have storage and structural roles)
-the function of a polysaccaride is determined by its sugar monomers and the postions of its glysodic linkages
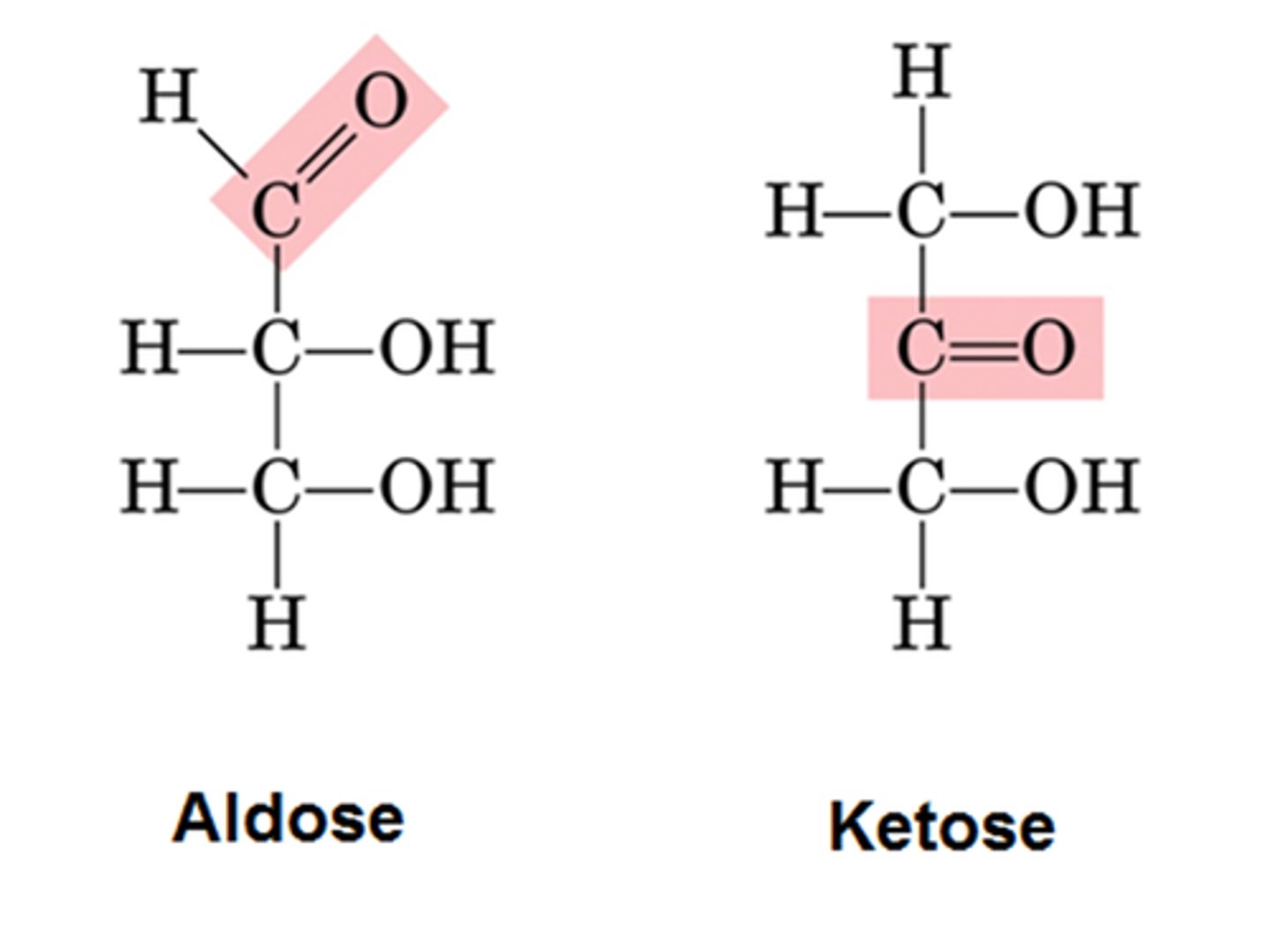
Given a linear form of a monosaccharide be able to distinguish the ones that are aldehydes vs ketones.
aldehydes have c double bonded to o slanted at the top while ketoses have it straight in the middle.
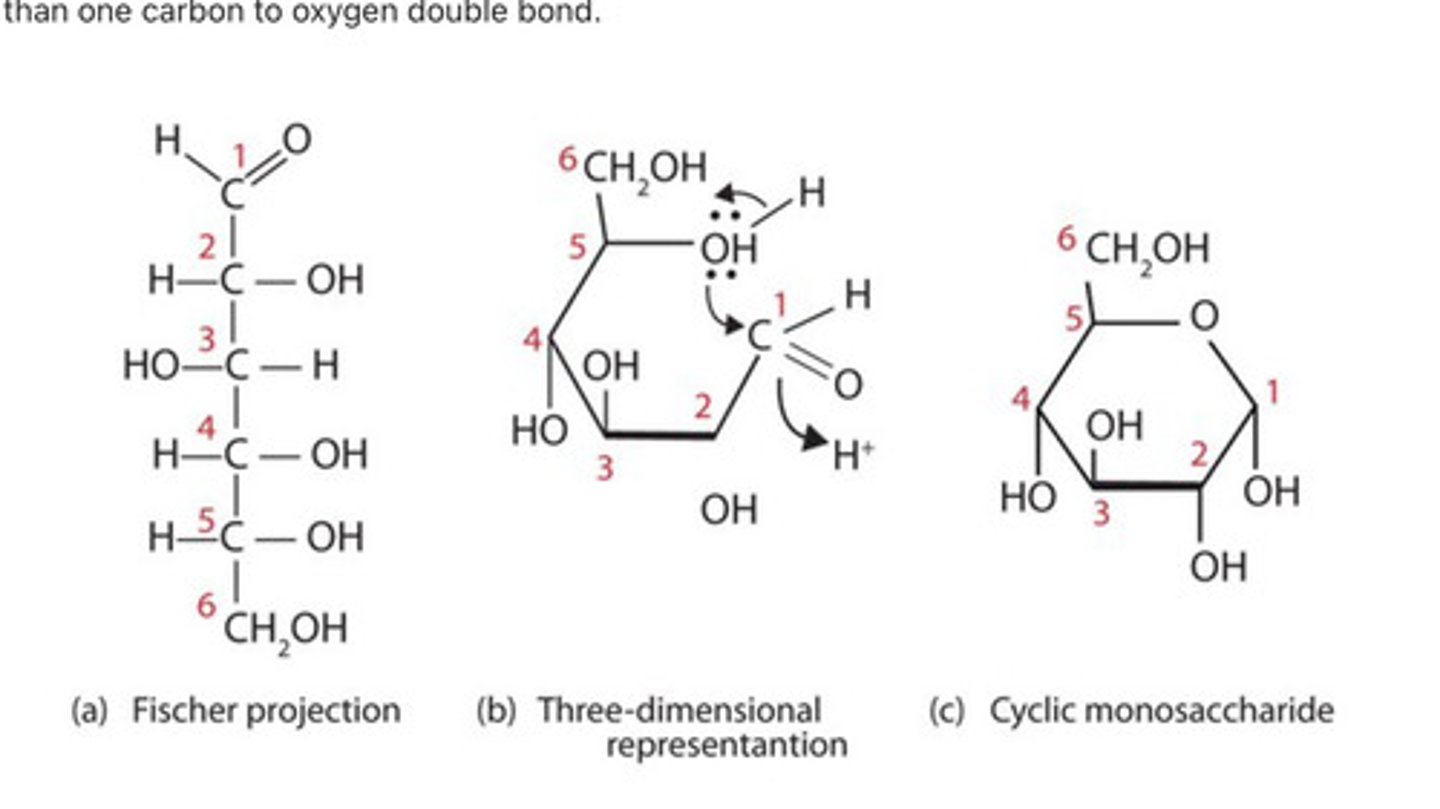
Be able to explain the conversion of the linear form of monosaccharides to ring form when present within the aqueous environment of a living cell.
rings are most stable form. in aqueous solutions many sugars form in rings under physiological conditions. the carbon 1 of he chain reacts with hydroxyl group of the carbon 5 in the Prescence of water.
https://www.google.com/search?q=how+polysacharide+chians+from+rings+animation&rlz=1C1CHZN_enUS931US931&oq=how+polysacharide+chians+from+rings+animation&gs_lcrp=EgZjaHJvbWUyBggAEEUYOTIJCAEQIRgKGKAB0gEJMTIyMzZqMGo5qAIAsAIA&sourceid=chrome&ie=UTF-8#fpstate=ive&vld=cid:0ba7ba26,vid:Ne2119IFpoQ,st:0
Glucose, fructose and galactose are isomers. Three disaccharides are found in food. Be able to state the monomers involved in the formation of common disaccharides in food Maltose (malt sugar), lactose (milk sugar) and sucrose (table sugar).
-maltose: glucose+glucose= maltose
-sucrose: glucose+fructose= sucrose
-lactose: galacacose+glucose=lactose
Be able to explain the term Glycosidic linkage. Which reaction produces this linkage or bond and which reaction breaks it?
it's the covalent bond between 2 monosaccharides. it's formed by dehydration synthesis and broken by hydrolysis
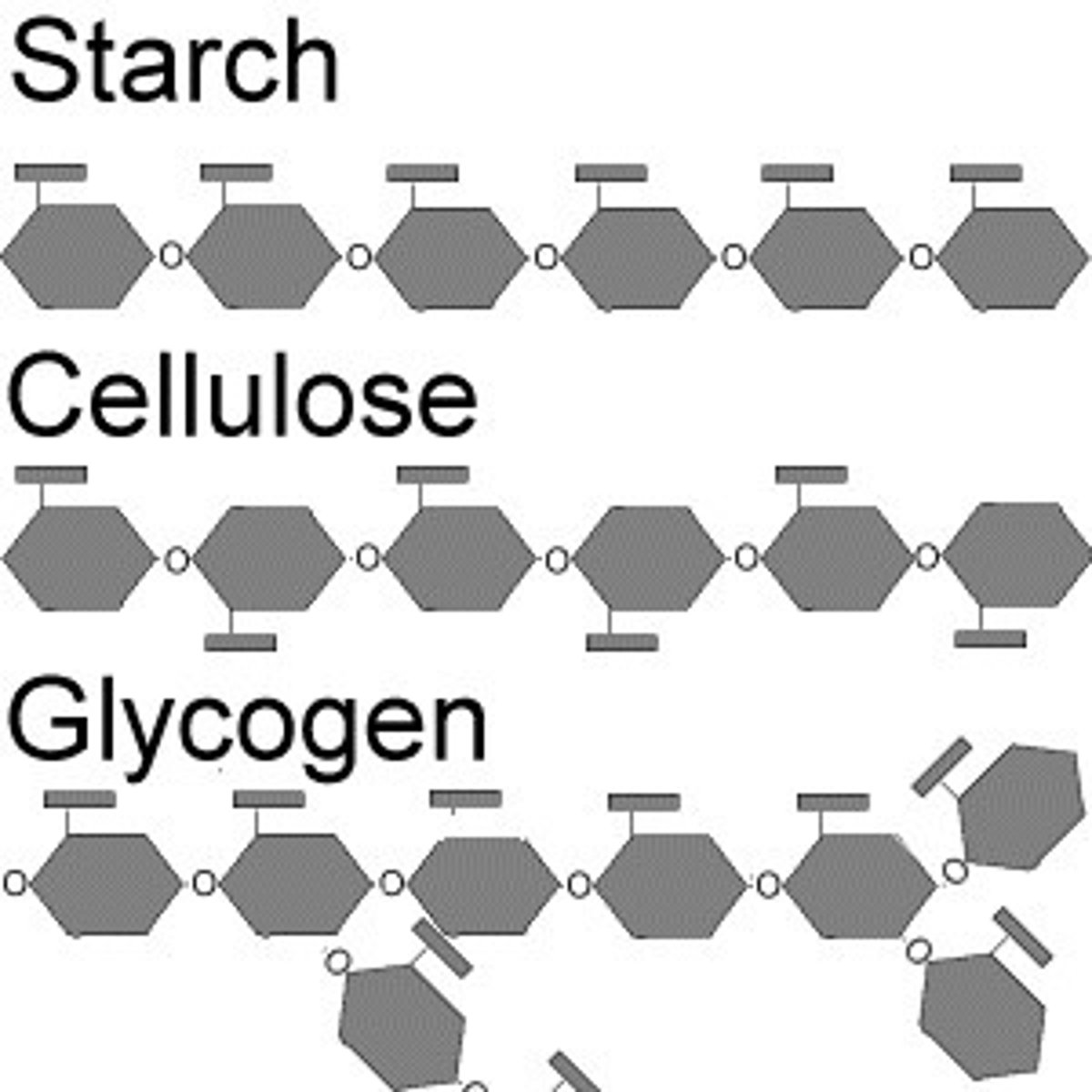
Be able to state which isomer of glucose is involved in forming the polysaccharides: starch, glycogen and cellulose and the nature of the glycosidic linkages in each case
storage polysaccharides:
- starch, consists of glucose monomers, plants store surplus starch in chloroplasts. simplest form of starch is amylose and amylopectin.
-glycogen, storage in animals, mainly stored in liver and muscles, hydrolysis of glycogen in these cells releases glucose when the demand for sugar increases
-Starch is a linear polysaccharide that has branched and unbranched parts amylose being unbranched and amyloceptin being branched
-Cellulose is a unbraced polysaccharide but every other ring is flipped.
structural polysaccharides: cellulose is a major component of plant cell walls
-Glucose in a ring form can have two different arrangements of the hydroxyl group (−OH) around the anomeric carbon (carbon 1 that becomes asymmetric in the process of ring formation). If the hydroxyl group is below carbon number 1 in the sugar, it is said to be in the alpha (α) position, and if it is above the plane, it is said to be in the beta (β) position.
glysodic linkages for cellulose are different.
2 ring forms alpha and beta
starch is alpha
cellulose is beta
alpha cant hydrolize beta
chitin is another strucural polysaccaride found in exoskeleton of arthopods also in cell walls of fungi
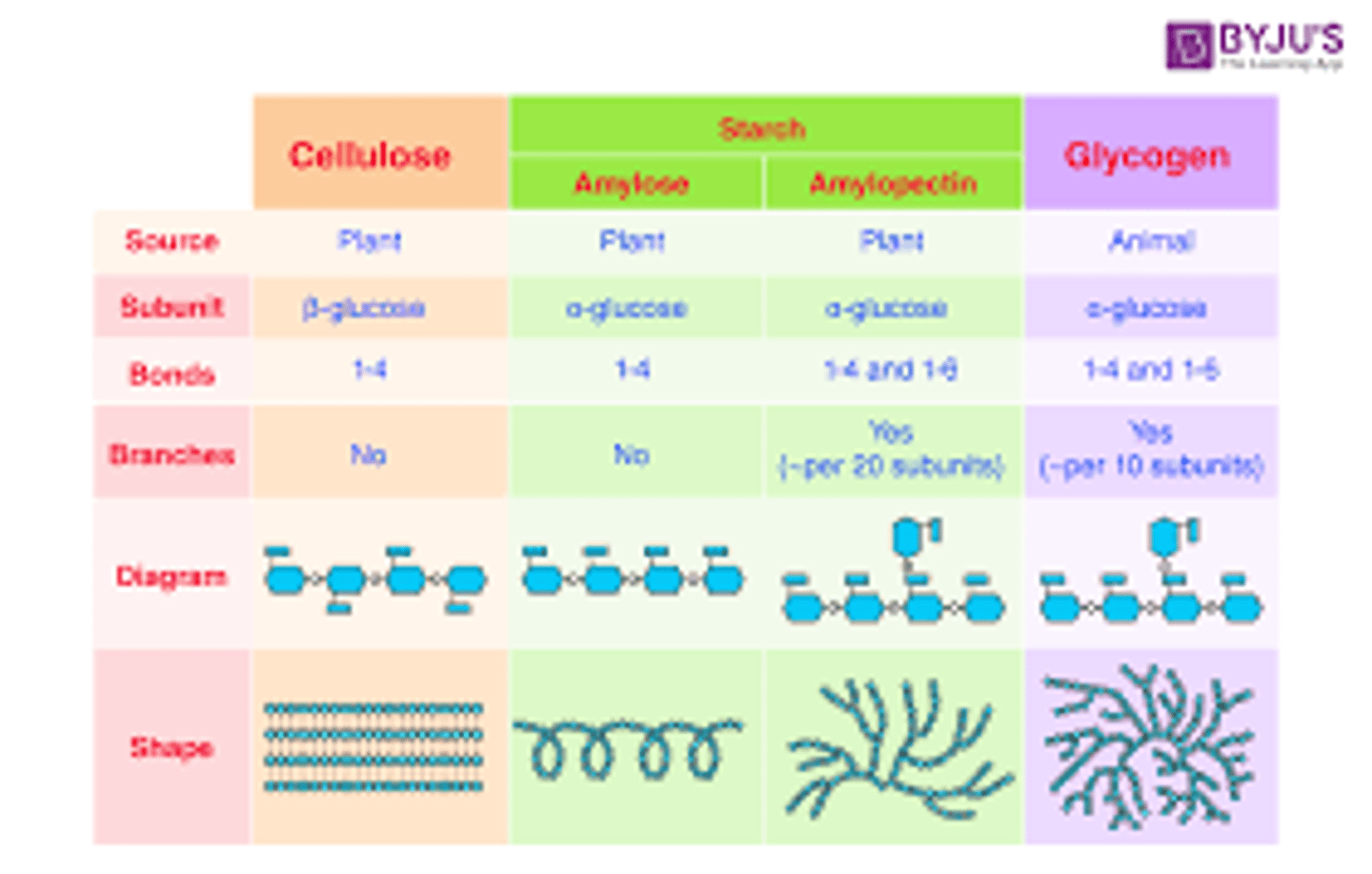
Given the well-studied polysaccharides: starch, glycogen, cellulose, chitin, be able to classify them as storage or structural and the kinds of living organisms in which they are found.
starch(plant) and glycogen(animal)= storage
cellulose(plant) and chitin(anthropod and fungi) = structural
Be able to explain the specific structures of starch, glycogen and cellulose (which isomer of glucose and the specific nature of the glycosidic bonds i.e. their numbering which describes the atoms of the monomers that are linked)
starch: alpha, 1 to 4 glycosidic bonds in amylose and 1 to 4 or 1 to 6 in amylopectin(branched)
glycogen: alpha-1,4 glycosidic bonds and 1-6 has both. (branched)
celluose=beta-1,4 linkage
The numbers are the placement of the glycosidic bonds.
Humans consume the above three polysaccharides in their diet. Which two of the above may be digested by humans and which one cannot be digested and why? Explain the advantages of having fiber in our diet.
humans can digest starch and glycogen but not cellulose. Cellulose is the carbohydrate that the humans cannot digest, it helps the digestive system, by helping in moving food along the intestines, as the fibre moves along the large intestine, bacteria feed on it and together the bacteria and fibre add bulk to the food.Dietary fiber increases the weight and size of your stool and softens it
Name the two forms of starch and which one is branched? Is cellulose branched? Is glycogen branched?
Starch consists of glucose molecules. It can occur in two forms: amylose and amylopectin. Amylose is a linear or straight-line polymer that scientists describe as amorphous or solid. Amylopectin forms a branched chain and is crystalline.
Cellulose is an example of an unbranched polysaccharide
Glycogen is a branched biopolymer consisting of linear chains of glucose
In which of the polysaccharides, is the arrangement of individual molecules parallel to each other as chains with inter-molecular hydrogen bonding between them. What consequence does this structure have on the function of this polymer in the cell wall?
cellulose because it is unbranced making it straight and parallel
cellulose fibers present in the plant cell wall is responsible for maintaining the shape and rigidity of plant cells.
In which of the above polysaccharides is the linear stretches of the polymer helical in nature.
Amylose consists of a linear, helical chains (starch)
How do cows digest cellulose (e.g. from grass) in their diet? How do termites digest wood?
Animals such as cows have anaerobic bacteria (does not require molecular oxygen for growth) within their digestive tracts which digest cellulose.
The termites masticate(Chewing) the wood to mechanically break it down, and the microbes chemically digest the wood into smaller molecules that the termites are able to absorb and utilize.
enzymes
speacialized macromolecules that speed up chemical reactions such as those that make or break down polymers
reaction that connects a monomer to another monomer is a _______________ reaction through a covalent bond.
condensation/hydrolysis
What are the three kinds of lipids found in living organisms and what commonproperty do they share? Which atoms are responsible for this specific property?
-fats, phospholipids, and steroids
-Insoluble in water
-Due to hydrocarbons that are non polar
Be able to state major functions of lipids in living cells
-storing energy, structural components of cell membranes, and form various messengers and signaling molecules within the body.
Be able to identify a fatty acid
-fatty acid consists of a straight chain of an even number of carbon atoms, with hydrogen atoms along the length of the chain and at one end of the chain and a carboxyl group (―COOH) at the other end. It is that carboxyl group that makes it an acid (carboxylic acid).
Be able to differentiate between a saturated fatty acid and unsaturated fatty acidbased on the presence of carbon-carbon single or carbon-carbon double bondswithin the hydrocarbon chain. Which atom is the carbon atom in the hydrocarbon chain of a saturated fatty acid "saturated with"?
-hydrocarbon chain contains a double bond it is unsaturated
-Saturated fatty acids are saturated with hydrogen since single bonds increase the number of hydrogens on each carbon
-saturated with hydrogen atoms
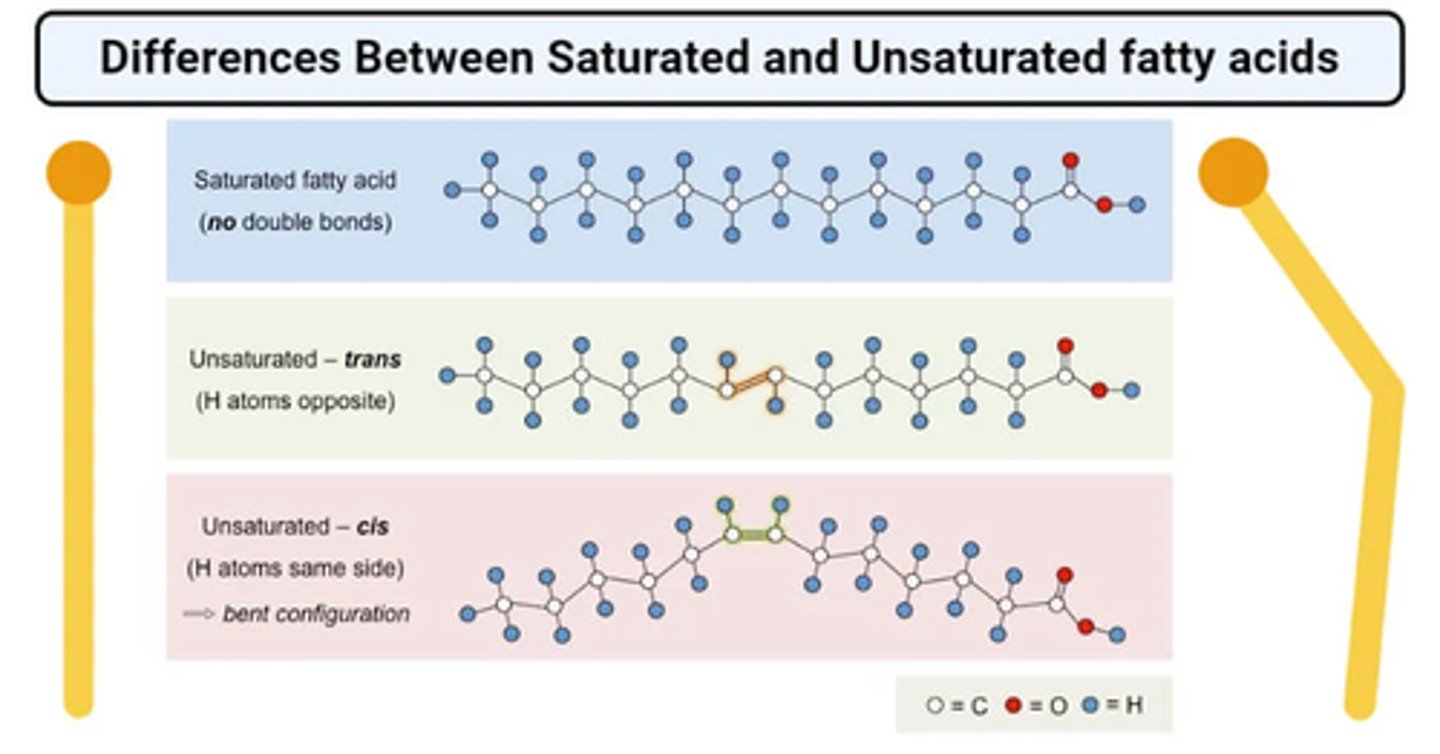
Which of the two possible configurations of the Carbon-carbon double bond iscommonly present in unsaturated fatty acid chains in nature? How does this doublebond affect the hydrocarbon tail?
-monounsaturated fats (one C double bond)
-polyunsaturated fats (multiple C double bonds)
-the double bonds cause the bends in the tails. Important for cell membranes so things can pass through.
What is the most common form of lipid that is used as an energy store in cells? What is this popularly referred to as?
Triglycerides are the most common type of fat in the body. They store excess energy from your diet.
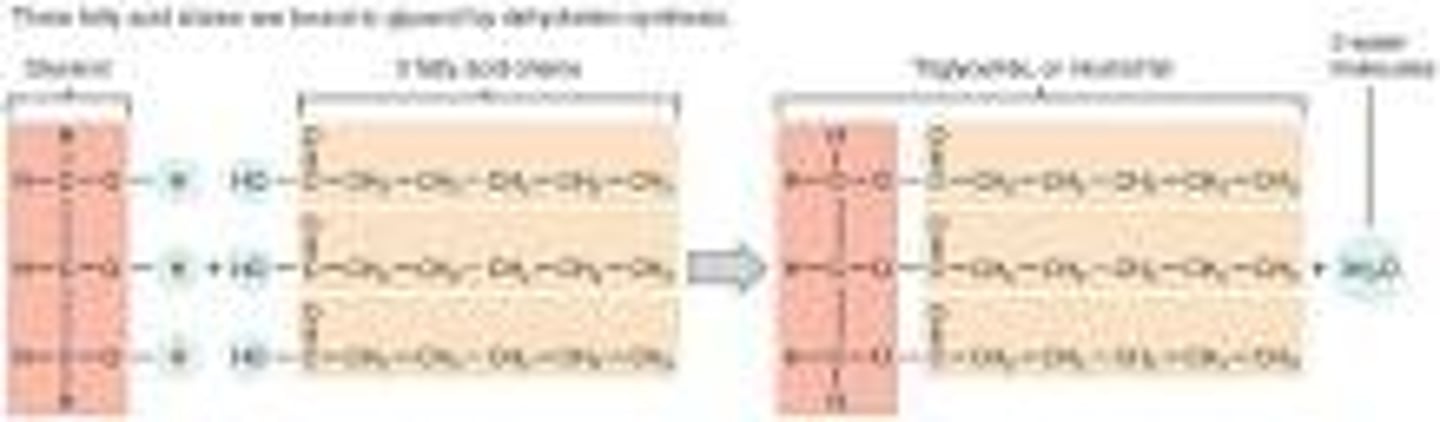
Be able to state the two different kinds of building blocks used to build tri-glycerides (generally referred to as fats). Be able to identify the dehydration reactions that occur between these building blocks to create triglycerides and the specific linkage created by these dehydration reactions. Is the triglyceride a macromolecule?
-Built by glycerol head and three fatty acid tails.
-Glycerol gives up hydrogen atoms from its hydroxyl groups at each bond, and the carboxyl group on each fatty acid chain gives up a hydroxyl group. A total of three water molecules are thereby released.
- bond that is fromed is a covalent bond called a ester bond (Esters are formed by the condensation reaction between an alcohol and a carboxylic acid.)
-no it is not made up of repeating subunits.
Explain why fats are insoluble in water
-contain hydrocarbons which are non polar and water is polar so they will not pair.
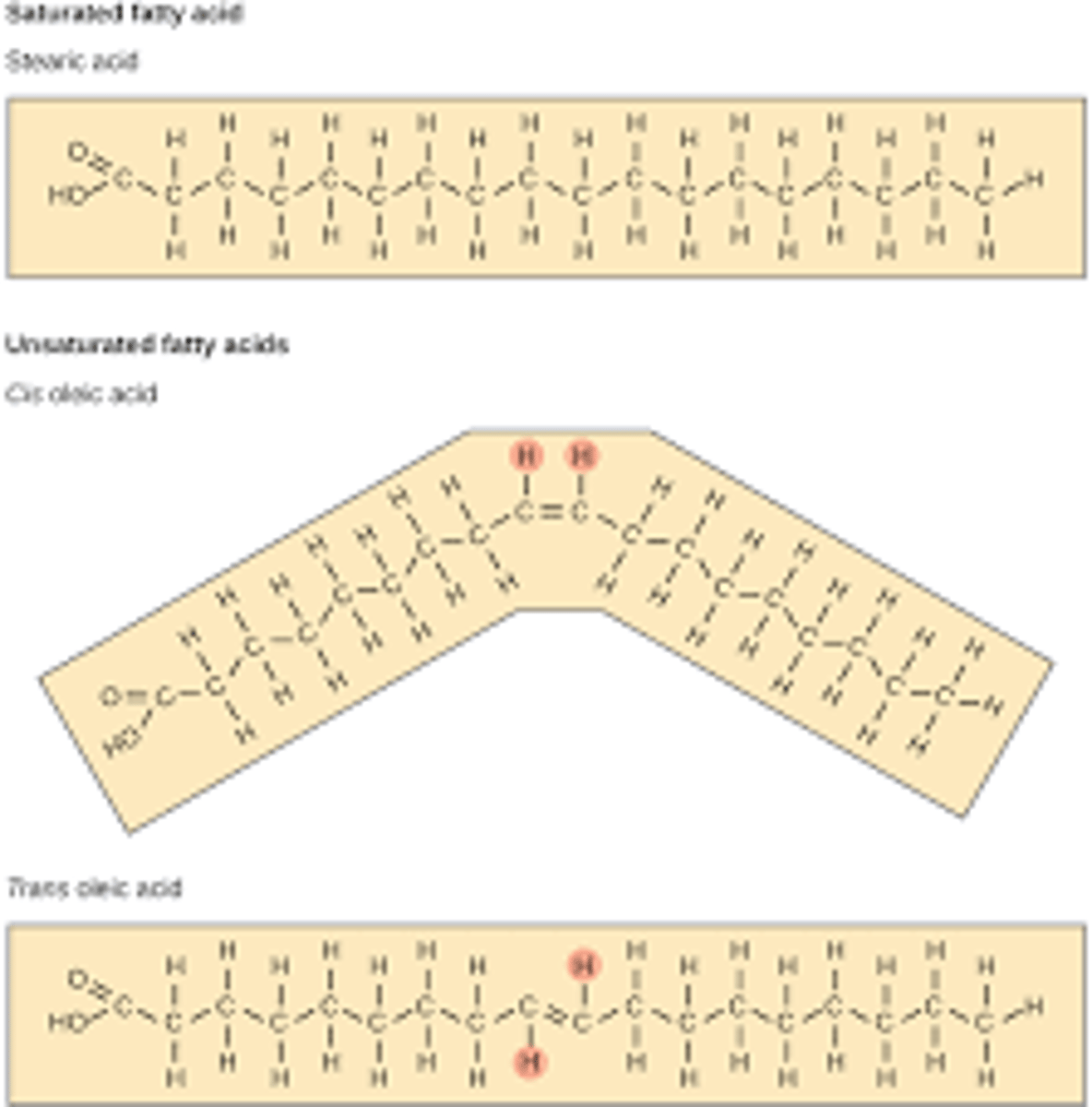
Be able to explain why (based on chemical structure) fats from animals such as cattle are solid at room temperature and why fats from plants and fishes (based on chemical structure) are usually liquid at room temperature.
-animal fats are saturated and has a straight compact structure due to every carbon being paired with as many hydrogens as possible
-plants fats are unsaturated and have bends which make it less compact cause it to be liquid at room temp.
Given the structure of triglyceride or the structure of a fatty acid that is used tomake a triglyceride, predict whether it is likely to be a solid or liquid at roomtemperature.
- fatty acid chain includes bends then liquid at room temp
-fatty acid chains don't include bends then are compact and solid at room temp.
Which process does the food industry use to convert vegetable oils which exist in liquid form at room temperature to a solid form such as in margarine and shortening? What forms as an additional by-product in the above reaction? Is this byproduct useful of harmful to health?
-Hydrogenation where manufacturers add hydrogen to liquid fat
-turns unsaturated fats into transfats to increase shelf life and make them solid.
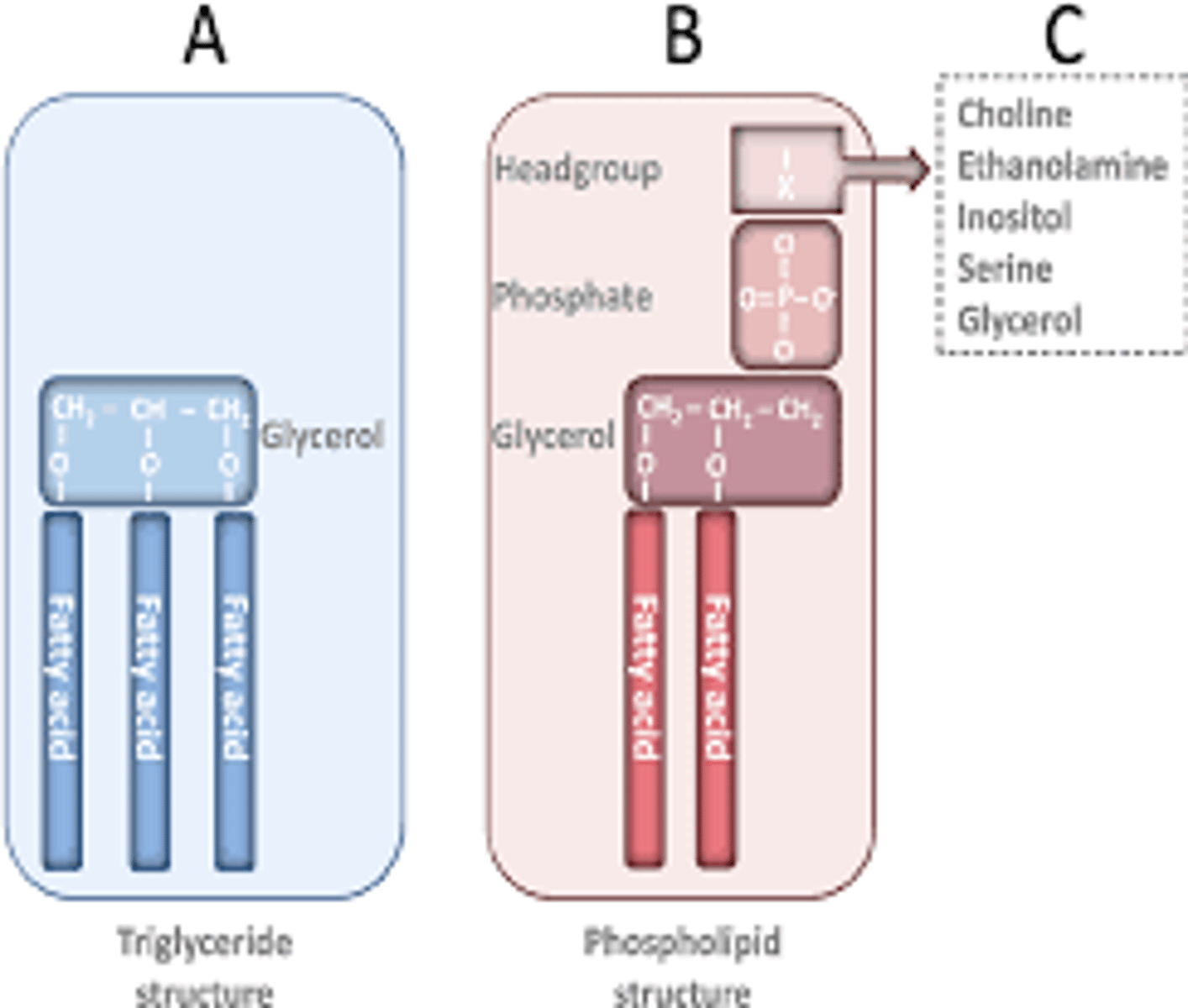
Be able to describe the structure of a phospholipid molecule. Which part of the phospholipid molecule is hydrophilic and which part is hydrophobic? What happens when phospholipids are mixed in water?
-made of glycerol, two fatty acid tails, and a phosphate-linked head group
-hydrophilic head and hydrophobic tail
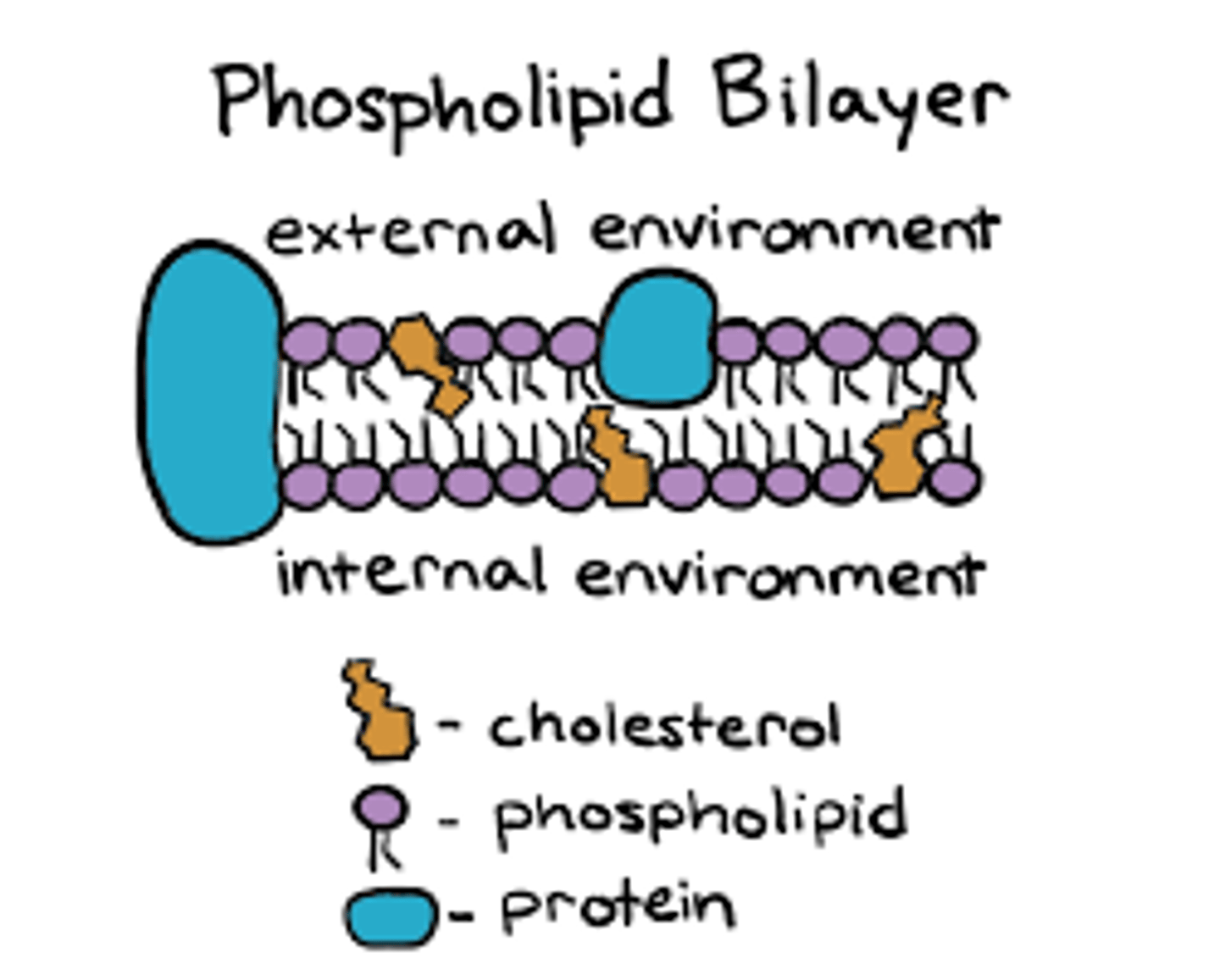
-phospholipids spontaneously form a double layer called a lipid bilayer. Philic heads face out and phobic tails point away from water. (this is hat creates cell membranes).
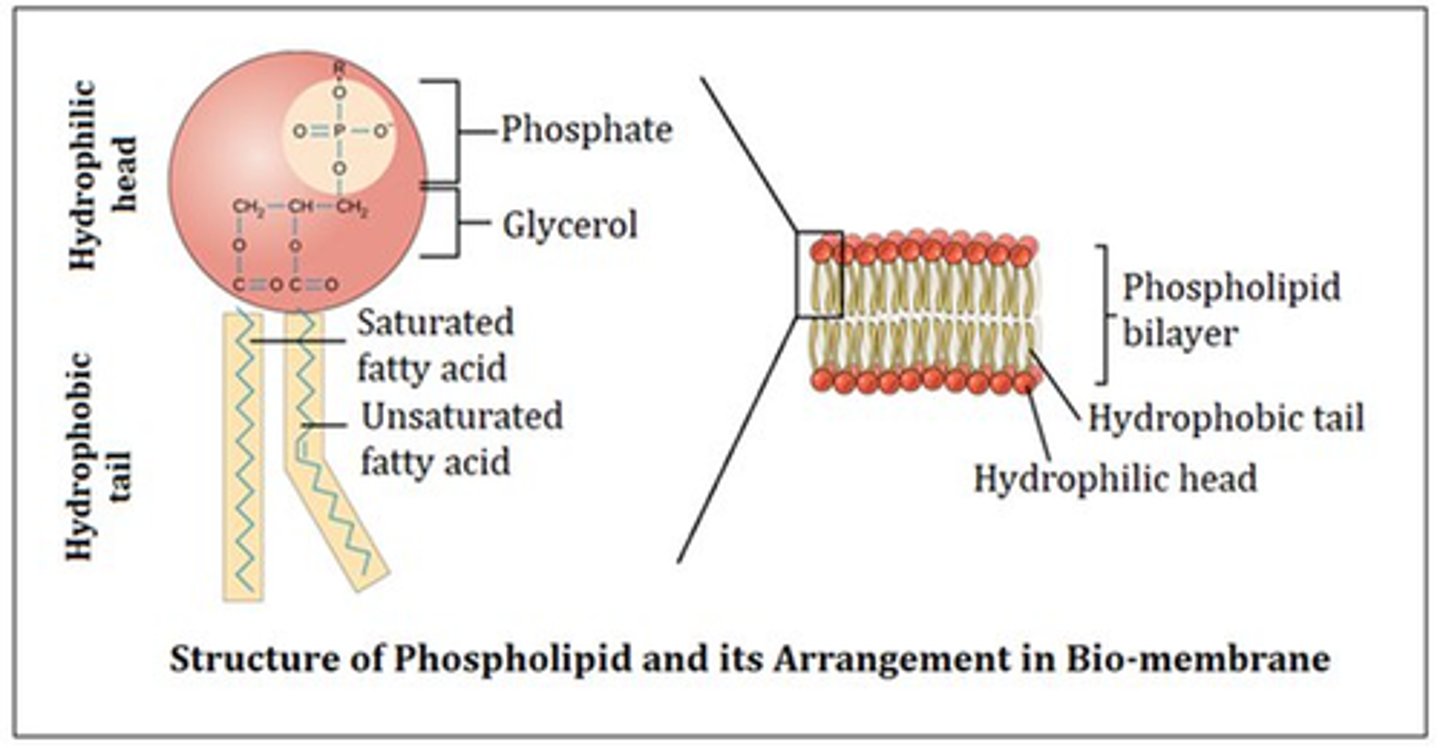
Be able to explain why phospholipids must form a bilayer to create boundaries such as the plasma membrane (also known as cell membrane). Why is a phospholipid monolayer not suitable to form the plasma membrane?
-tails are severely hydrophobic so they must create bilayer to still allow things in and out of the cell.
-if there was only one layer active transport would not be possible because the tails would not be permeable.
Be able to compare and contrast structure of Fats (triacylglycerols) with structure of phospholipids.
-TRIacyglycerols have 3 tails and not phosphate group
-PHOSPHOlipids have a phosphate group attached to glycerol head and only 2 tails one being bent.
Be able to explain the general structure of steroids. Given the structure of a molecule, be able to identify it as a steroid. Be able to explain why steroids fall under the category of lipids (hint: look at the majority of atoms that make up cholesterol for example).
-steroids have a fused ring structure
-grouped with them because they are also hydrophobic and insoluble in water.
-All steroids have four linked carbon rings, and many of them, like cholesterol, have a short tail. Many steroids also have the -OH functional group, and these steroids are classified as alcohols called sterols.
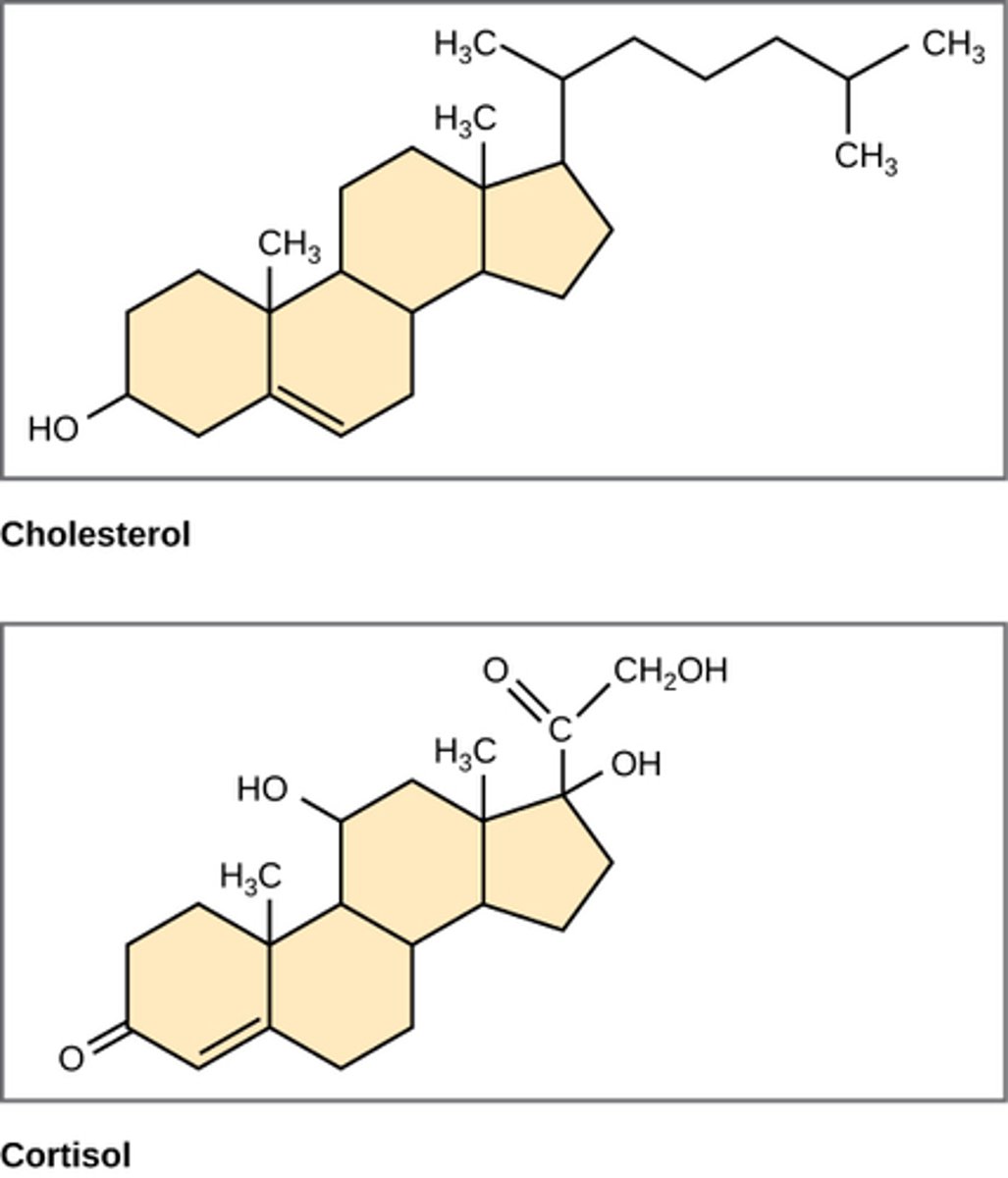
What are some of the other steroids that are made from the precursor steroid cholesterol? What are the known functions of the different derivatives? Is cholesterol also present in the plasma membrane of animal cells?
-precursor to vitamin D, testosterone, estrogen, progesterone, aldosterone, cortisol, and bile salts.
-they function as hormone's or reduce inflammation/maintain fluidity of cell membrane
-yes provides cell membrane structural integrity and fluidity
precursor
In biochemistry, the term "precursor" often refers more specifically to a chemical compound preceding another in a metabolic pathway, such as a protein precursor.
Steroid
A type of lipid characterized by a carbon skeleton consisting of four rings with various functional groups attached. A steroid is a biologically active organic compound with four fused rings arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and as signaling molecules.
derivative
A derivative is a compound that is synthesised from an identical compound by a chemical reaction with the replacement of one atom or group of atoms. For example - Ester is a derivative of Carboxylic acid.
Be able to explain the building blocks that constitute proteins and how many such building blocks are known for proteins. Be able to explain how diversity of protein arises.
-built from a set of twenty amino acids
-diversity of proteins is caused due to the varying sequence resulting from alternating proportions of amino acids.
-amino acid sequence determines protein structure and structure dictates biochemical function.
Be able to explain that every polypeptide has a specific sequence of amino acids arranged in a particular order
- The steps of the DNA ladder are made up of four different nitrogenous bases.
-sequence and pair of bases dictate the 3-D conformation the fold protein will have.
Polypeptide
A polypeptide is an unbranched chain of amino acids.
Chromosomes
a threadlike structure of nucleic acids and protein found in the nucleus of most living cells, carrying genetic information in the form of genes.
(really long strand of DNA)
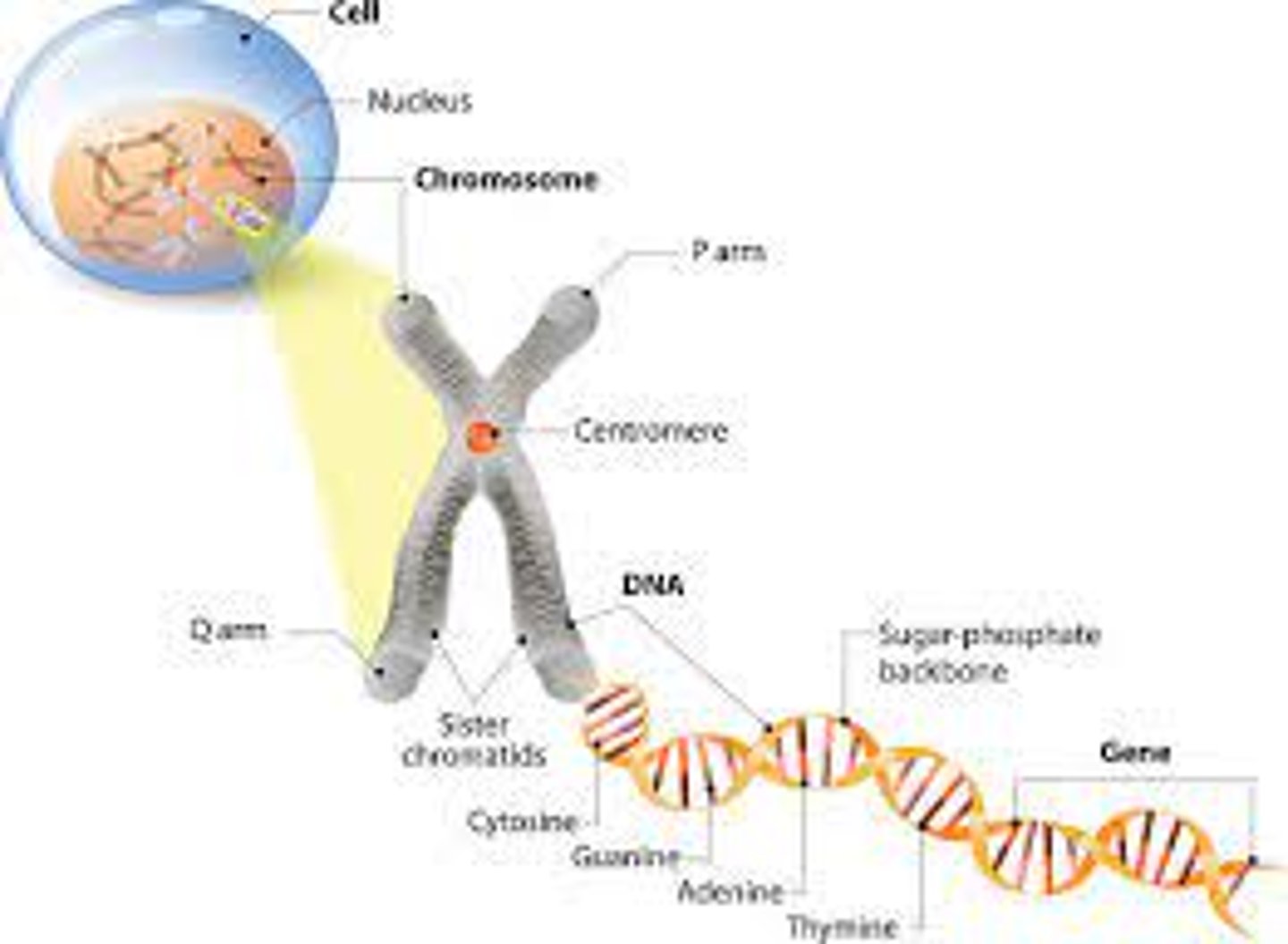
DNA
A complex molecule containing the genetic information that makes up the chromosomes. is twisted and that twisted shape is the double helix.
Steps of the ladder are made of four bases.
Genes
DNA segments that serve as the key functional units in hereditary transmission.
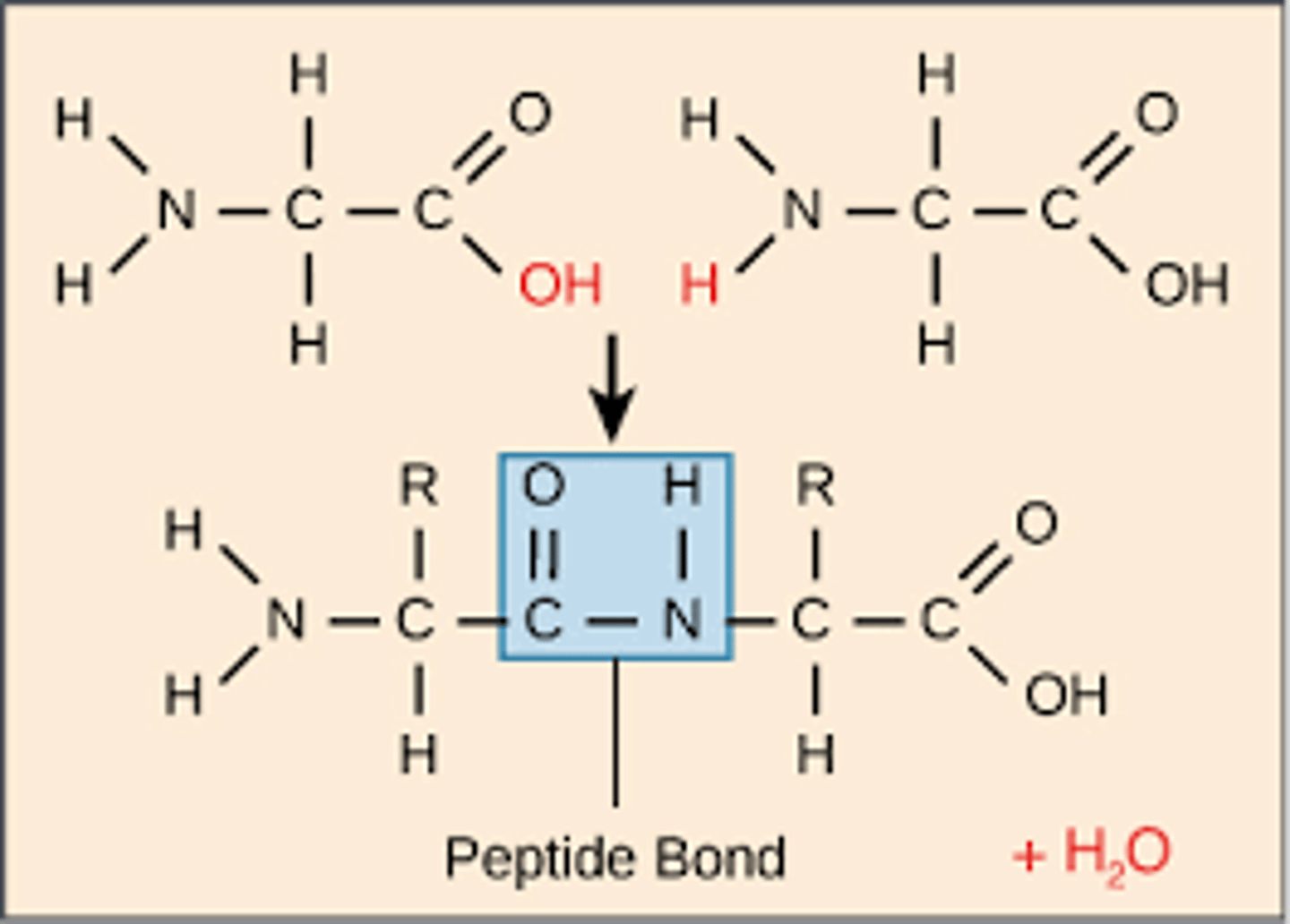
Be able to name and write the formula for the bond that connects amino acids in a polypeptide. What is the nature of the reaction that makes this bond: dehydration or hydrolysis?
https://www.khanacademy.org/test-prep/mcat/biomolecules/amino-acids-and-proteins1/v/peptide-bond-formation-and-cleavag
-bond that connects amino acids in a polypeptide = peptide bond.
-formula for a peptide bond is (−CO−NH−).
-A peptide bond is a covalent chemical bond that joins two amino acids by removing a water molecule (H2O) from an amino group (-NH2) of one amino acid and a carboxyl group (-COOH) of the adjacent amino acid. This reaction produces two amino acids joined by a peptide bond and a water molecule (H2O). The two joined amino acids are called a dipeptide.
e
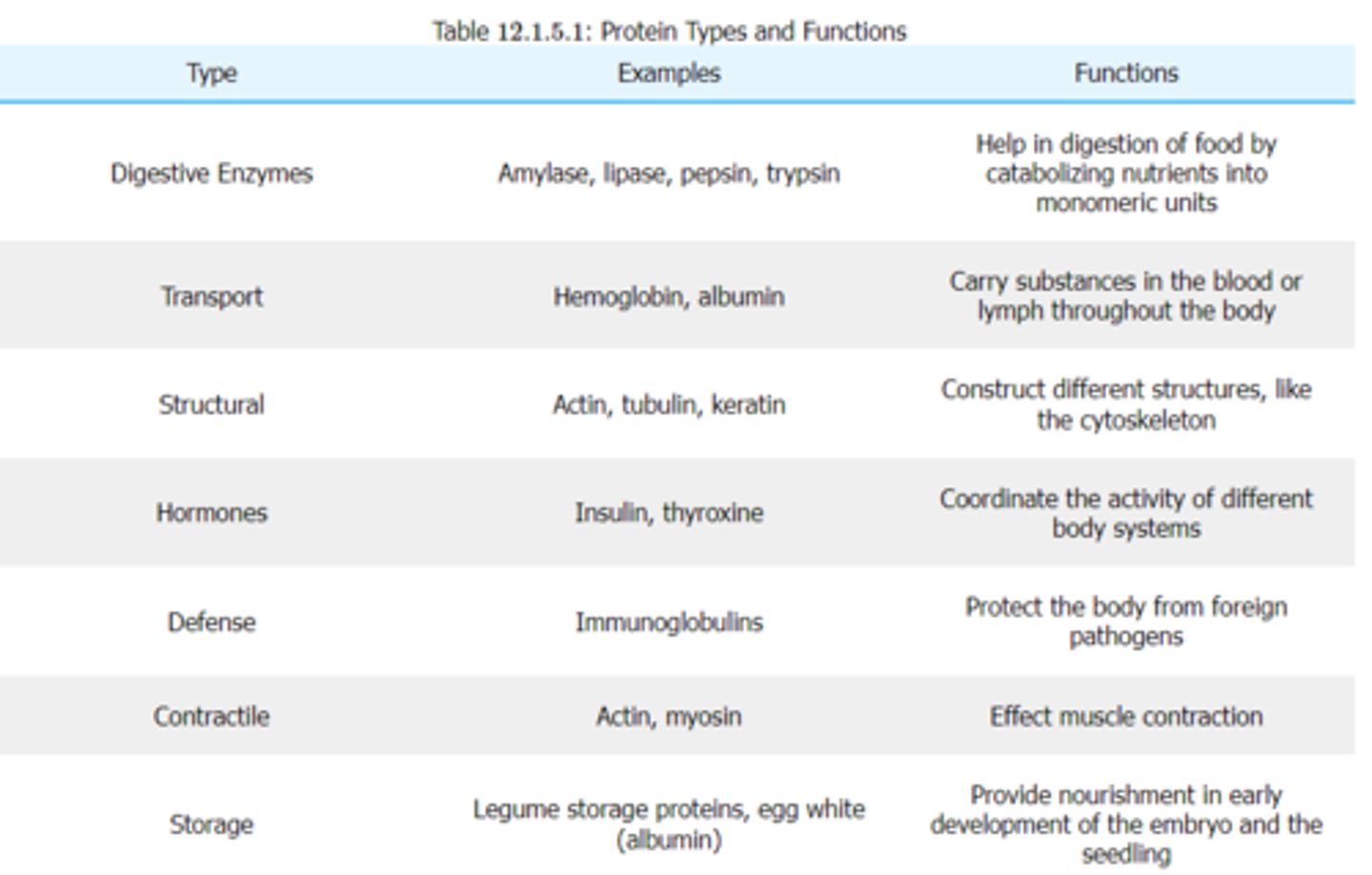
Enumerate various functions that are associated with proteins.
acting as enzymes and hormones, maintaining proper fluid and acid-base balance, providing nutrient transport, making antibodies, enabling wound healing and tissue regeneration, and providing energy when carbohydrate and fat intake is inadequate.
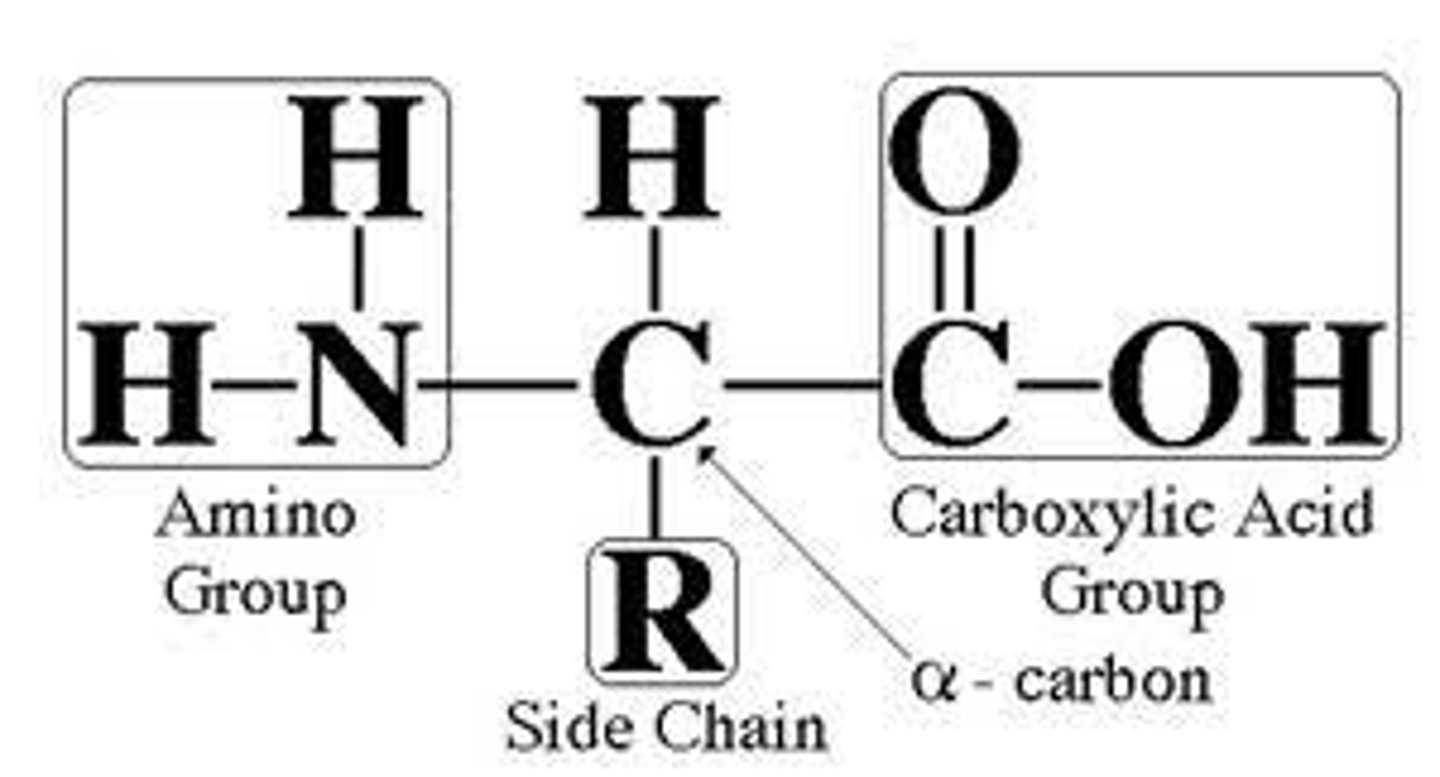
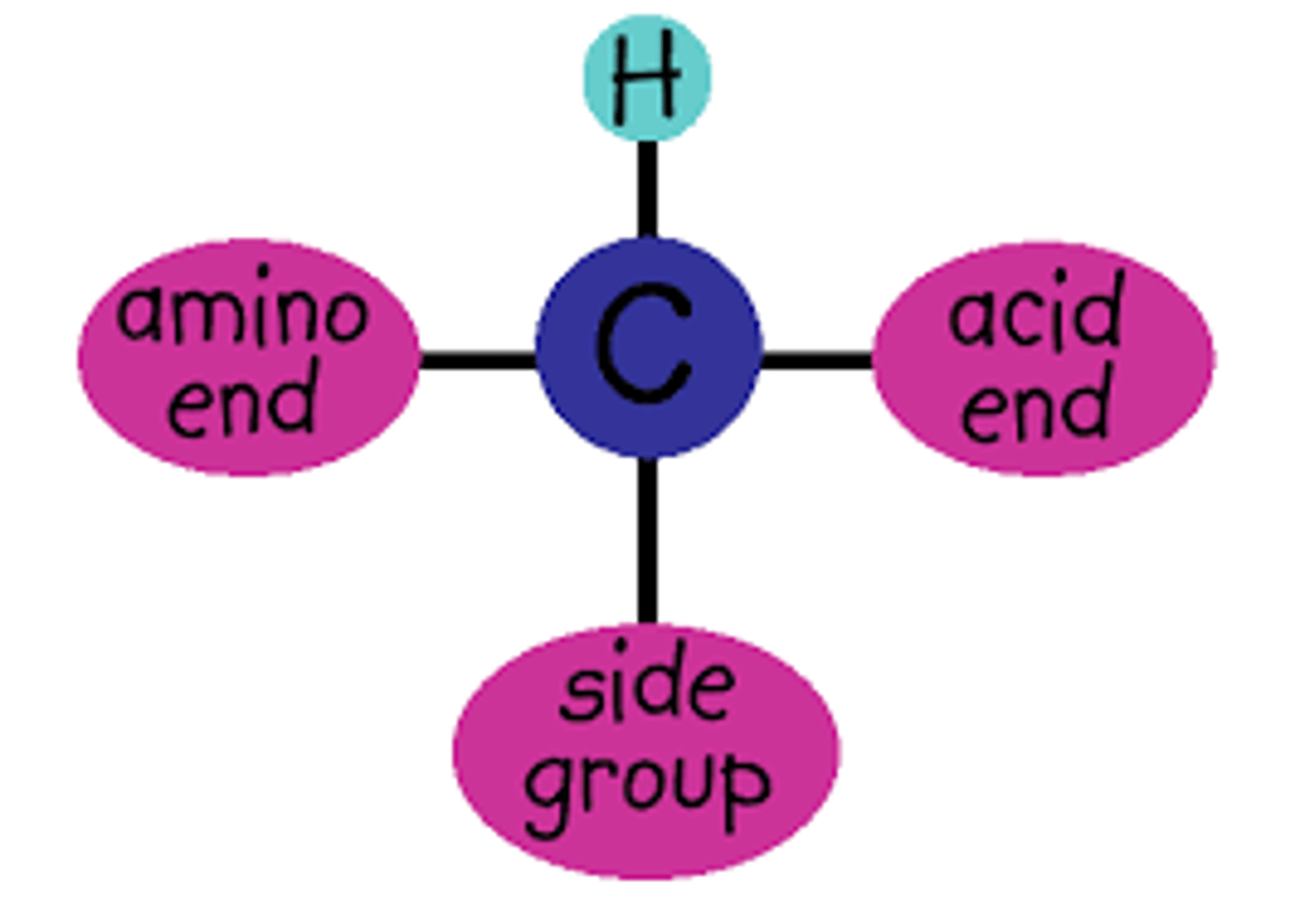
Be able to draw the general structural formula of an amino acid. What is R in such a structure. At what position in this general formula does kind of one amino acid differs from another kind of amino acid? Which two functional groups are present in every amino acid. Which group is acidic and which is basic?
-Formula for amino Acid =
R - C - H -NH2 - COOH
-R group differs between amino acids.
IT gets its name because it is composed of amine group (H2N) and a carboxylic Acid group COOH.
-carboxylic ACID is acidic
-Amine group is basic
-R group is attached to the alpha carbon.
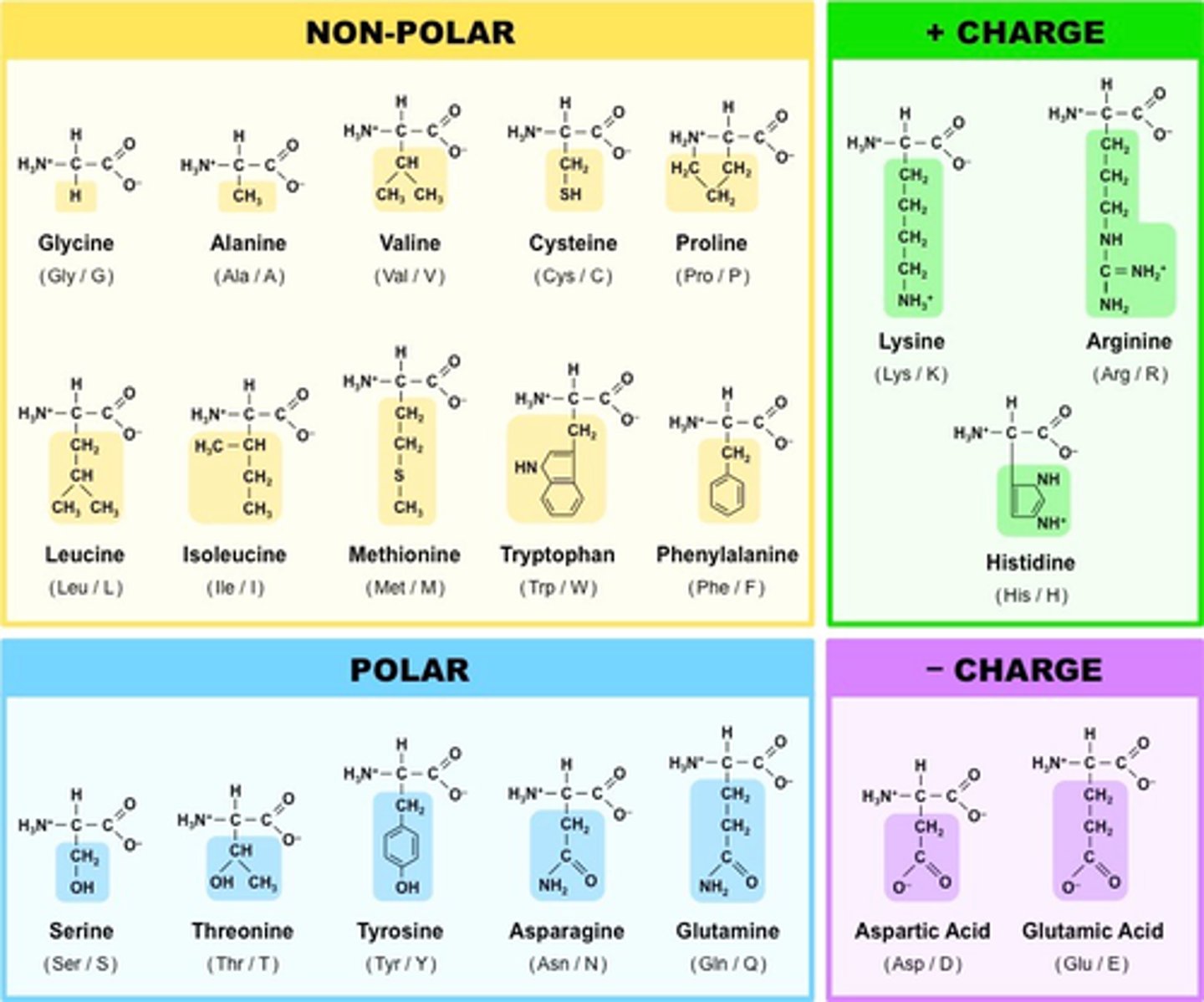
Be able name the three major classes of amino acids based on the property of their R-groups. Given the name of an amino acid be able to place them in one of the three groups. Given the structure of an amino acid be able to classify it into one of the three categories.
https://www.youtube.com/watch?v=BLj-7pYAyws&ab_channel=RealChemistry
-(1) those with nonpolar R group, (2) those with uncharged polar R groups, and (3) those with charged polar R group.
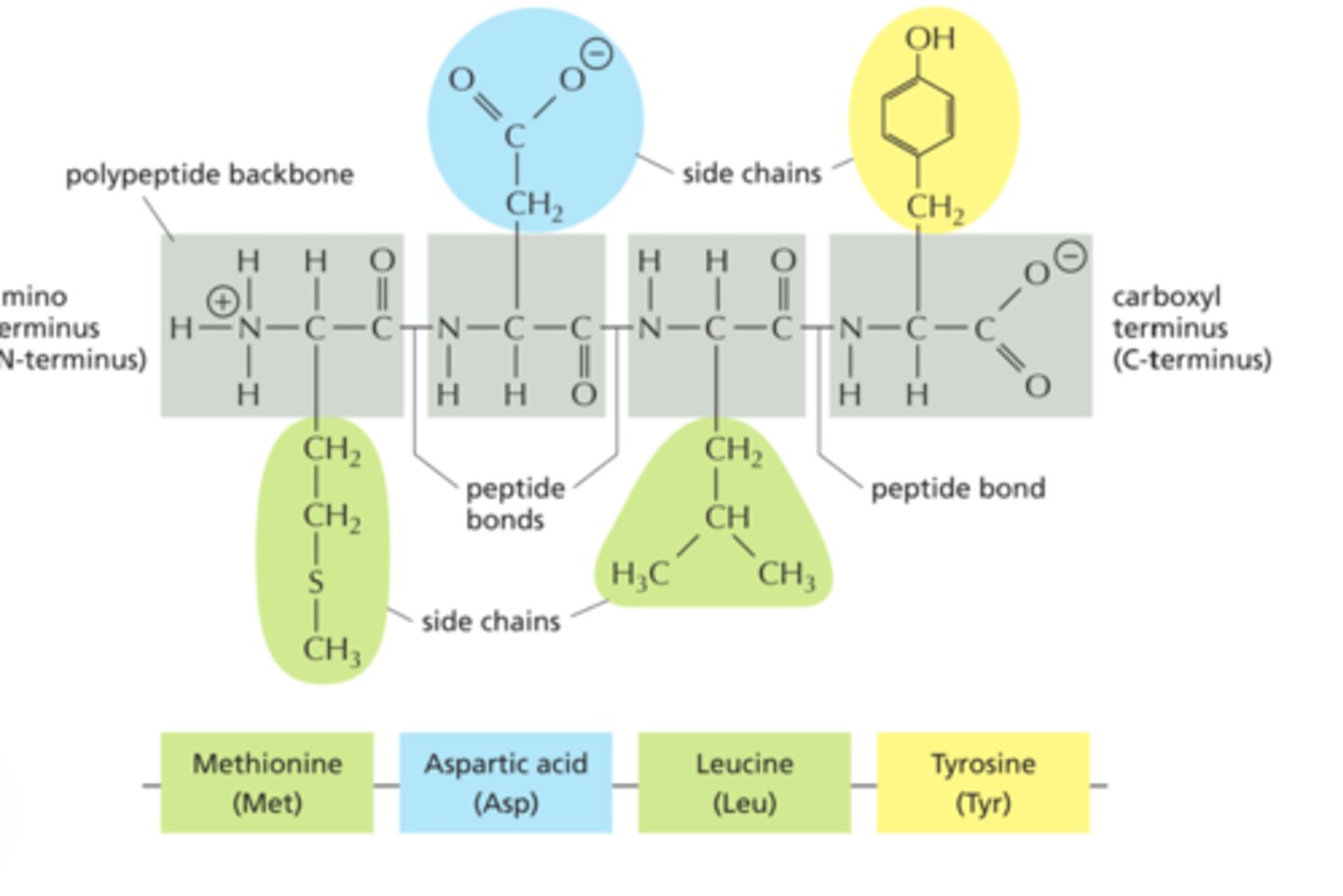
Be able to list the properties of the polypeptide backbone.
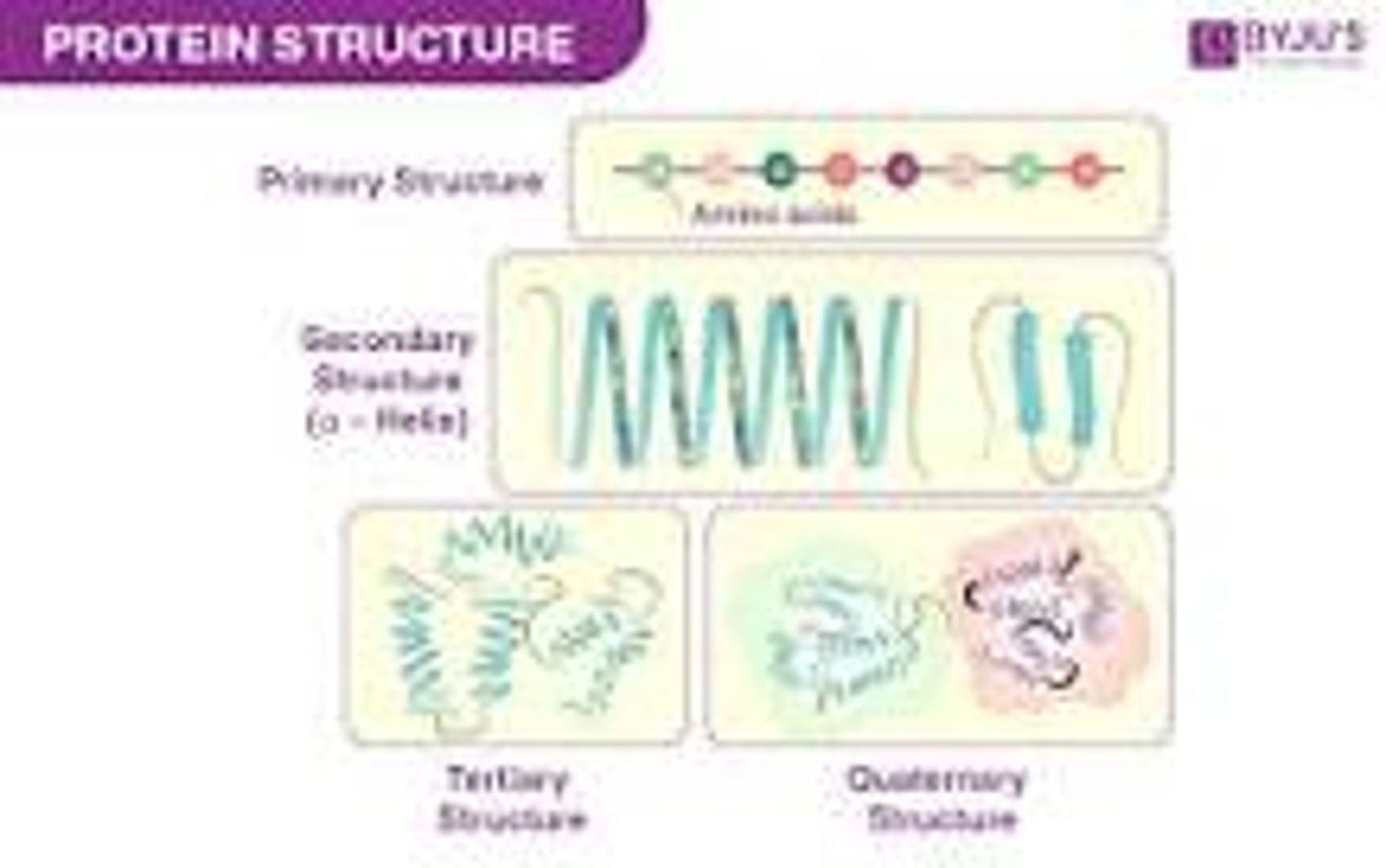
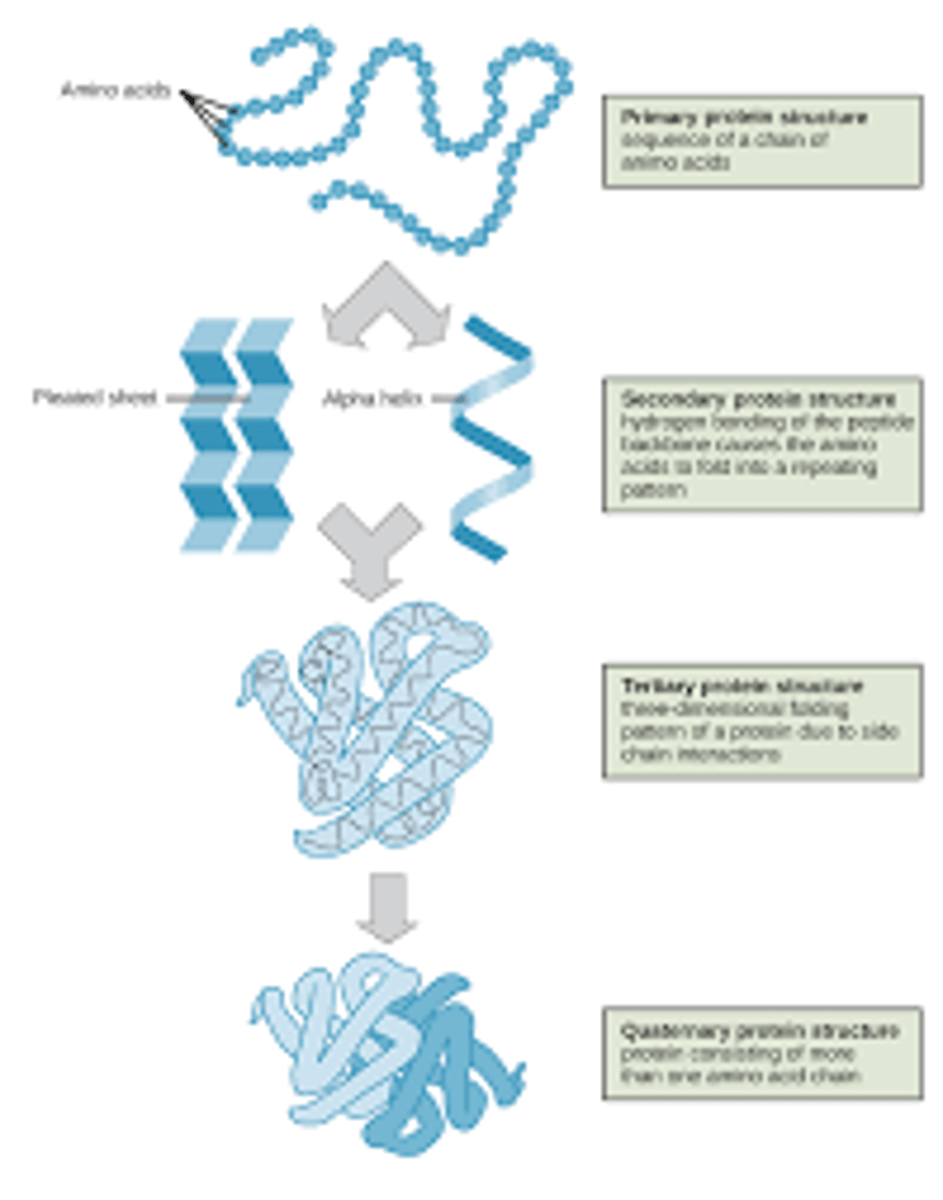
-known as the primary structure of the protein.
-protein fold and twist, forming into the secondary structure, which is rigidified by hydrogen bonding between the carbonyl oxygens and amide hydrogens in the backbone, i.e. C=O---HN.
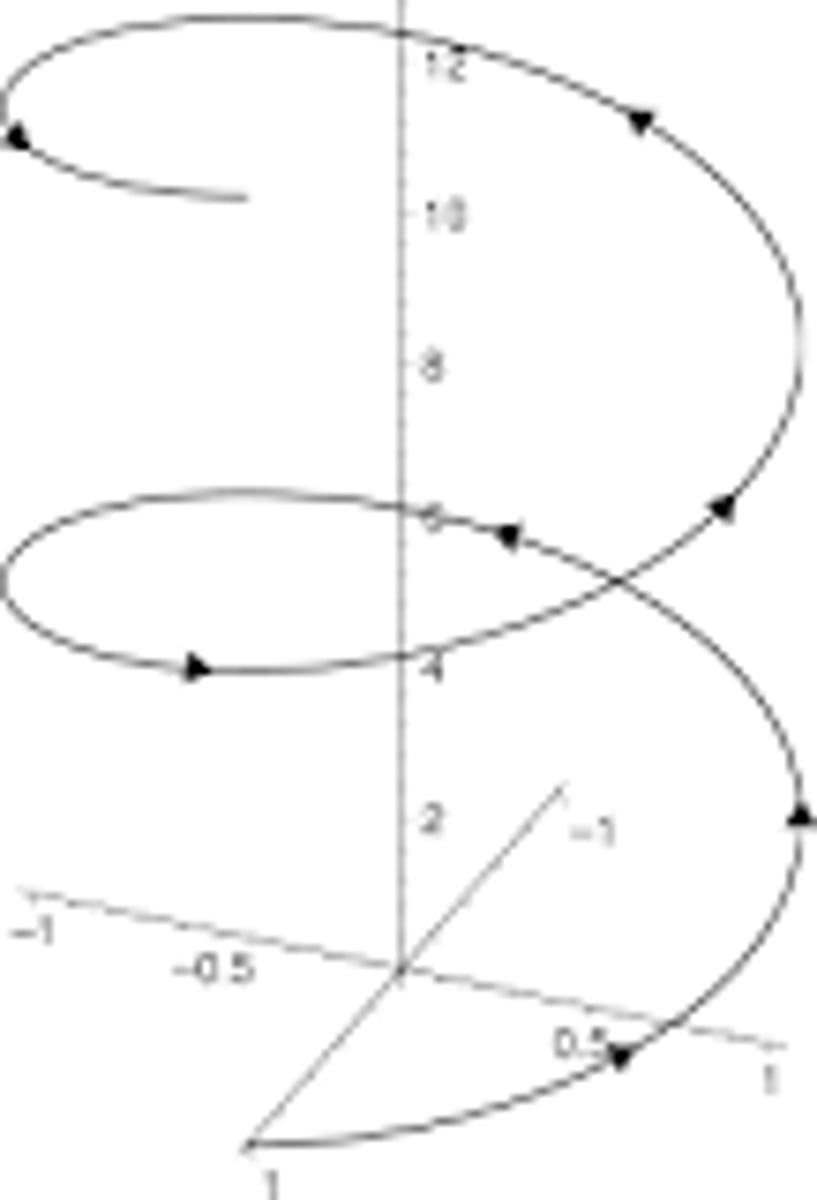
Helix
is a type of smooth space curve with tangent lines at a constant angle to a fixed axis. Helices are important in biology, as the DNA molecule is formed as two intertwined helices, and many proteins have helical substructures, known as alpha helices. The word helix comes from the Greek word ἕλιξ, "twisted, curved".
What determines the primary structure of a protein? Where is this information encoded in the first place in the cell?
-Polypeptide backbone
-Encoded in DNA in the nucleus
(image is for visualization of a polypeptide chai).
Name the nature of bond involved in the primary structure of a polypeptide
-onsists of amino acids joined by peptide bond
-Peptide bonds are between the alpha-carboxyl of one amino acid, and the alpha-amine of the next amino acid. A peptide bond is an example of an amide bond
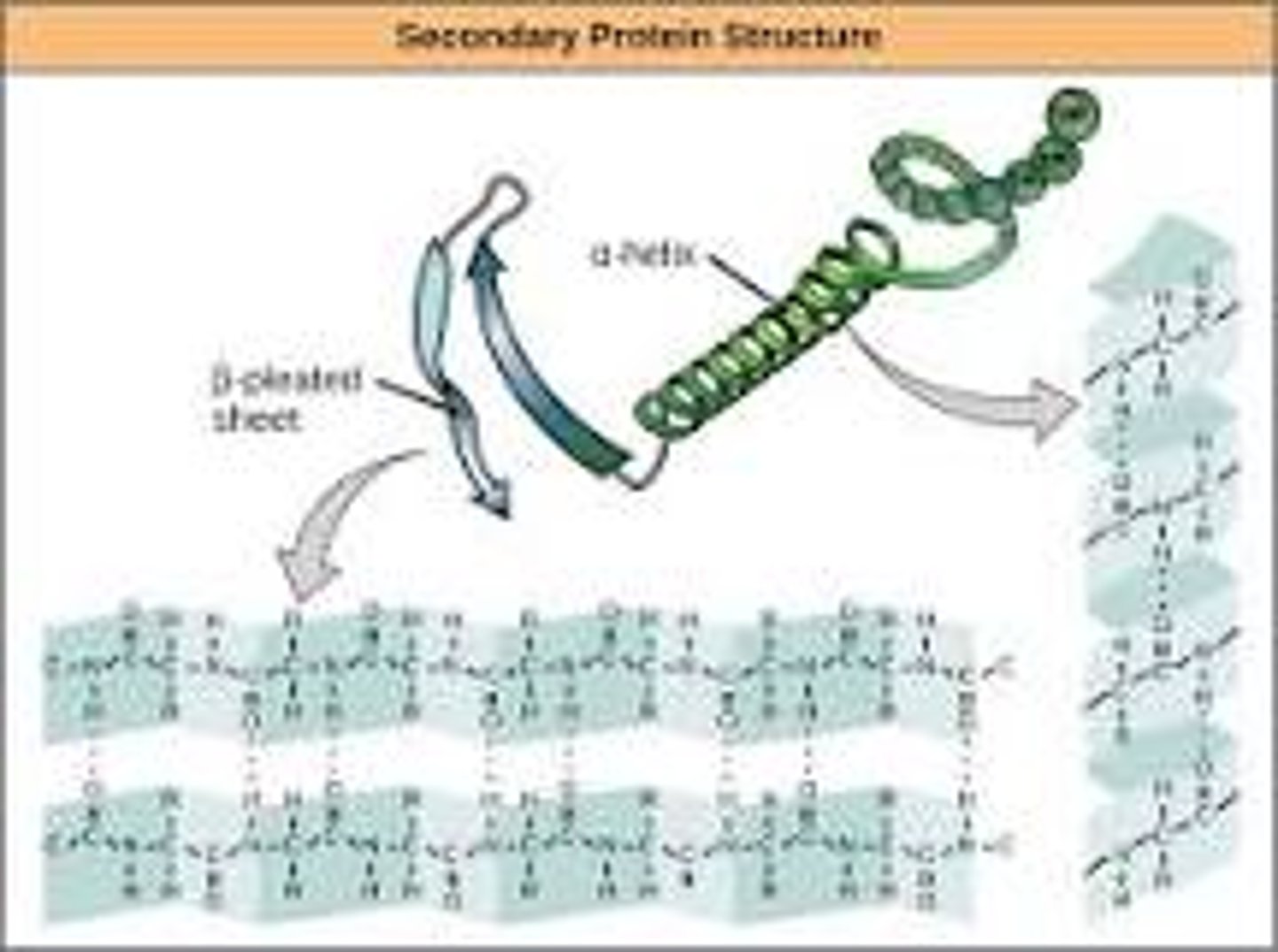
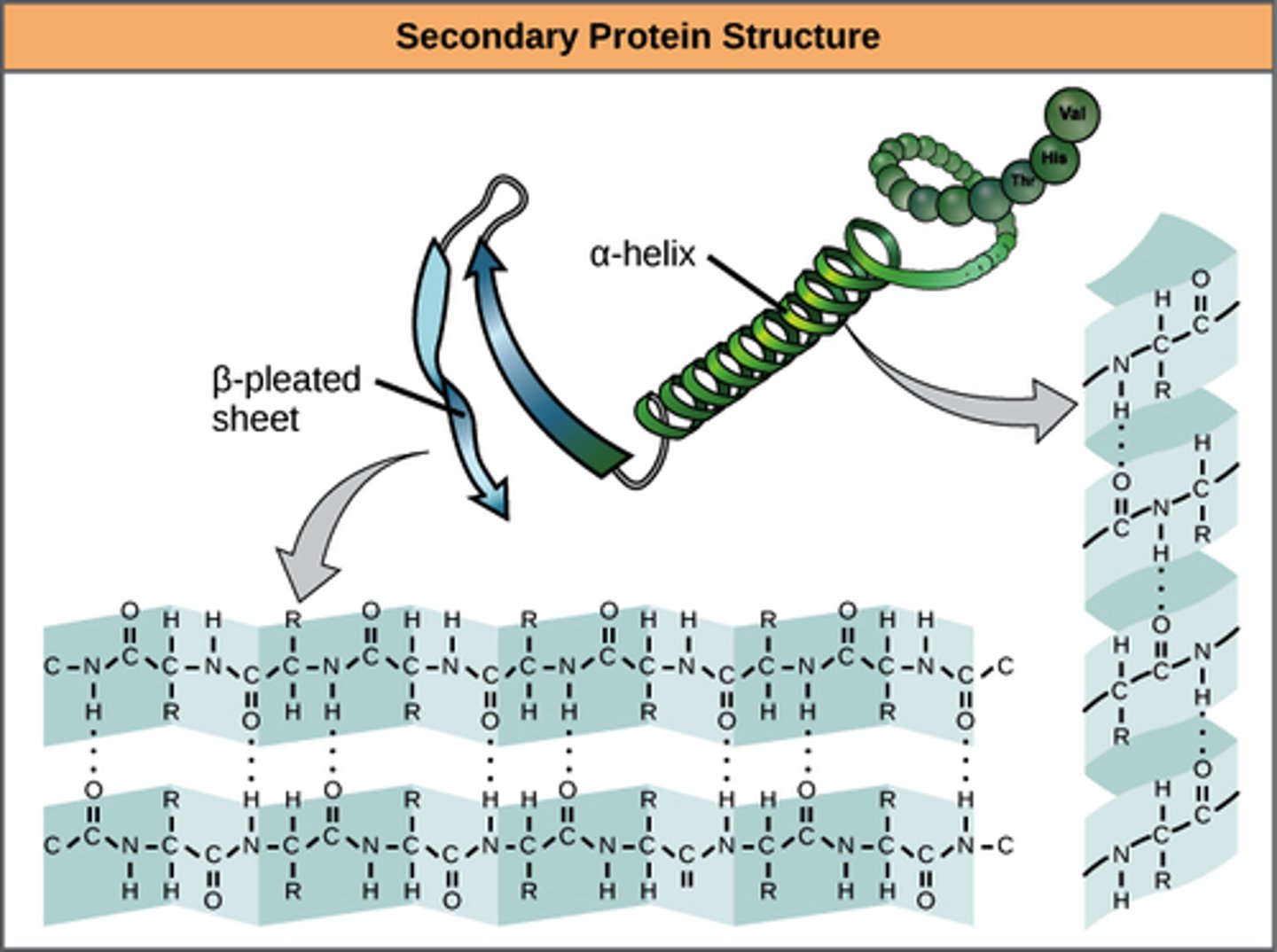
What is the secondary structure of a protein? What is the nature of the bonds that are involved in the formation of the secondary structure and does it involve the backbone atoms or does it involve the R-groups?
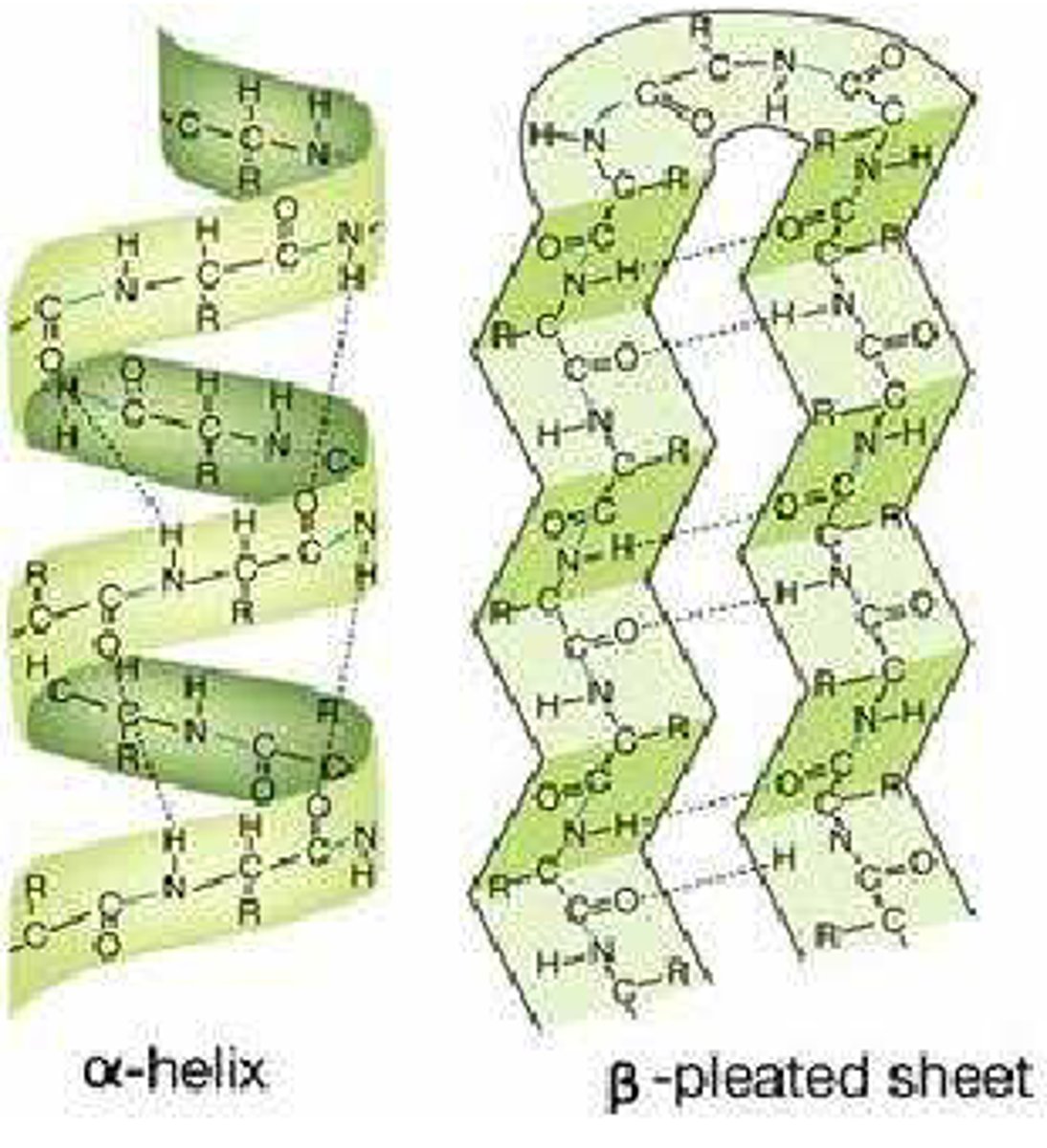
-the 3D form of LOCAL segments of proteins, HELD TOGETHER BY HYDROGEN BONDS BETWEEN THE AMINO HYDROGEN AND CARBOXYL OXYGEN OF THE BACKBONE. The two most common secondary structural elements are ALPHA helices and BETA SHEET, though beta turns and omega loops occur as well.
-The backbone just refers to the polypeptide chain apart from the R groups – so all we mean here is that secondary structure does not involve R group atoms.
Given the diagram of a secondary structure be able to recognize it as an alpha helixor a beta-sheet.
-In an α helix, the carbonyl (C=O) of one amino acid is hydrogen bonded to the amino H (N-H) of an amino acid that is four down the chain.
(E.g., the carbonyl of amino acid 1 would form a hydrogen bond to the N-H of amino acid 5.)
-In the alpha helix, the bonds form between every fourth amino acid and cause a twist in the amino acid chain. In the β-pleated sheet, the “pleats” are formed by hydrogen bonding between atoms on the backbone of the polypeptide chain.
Tip about amino acids
-change in the gene's DNA sequence may lead to a change in the amino acid sequence of the protein. Even changing just one amino acid in a protein's sequence can affect the protein's overall structure and function.
-For instance, a single amino acid change is associated with sickle cell anemia, an inherited disease that affects red blood cells.
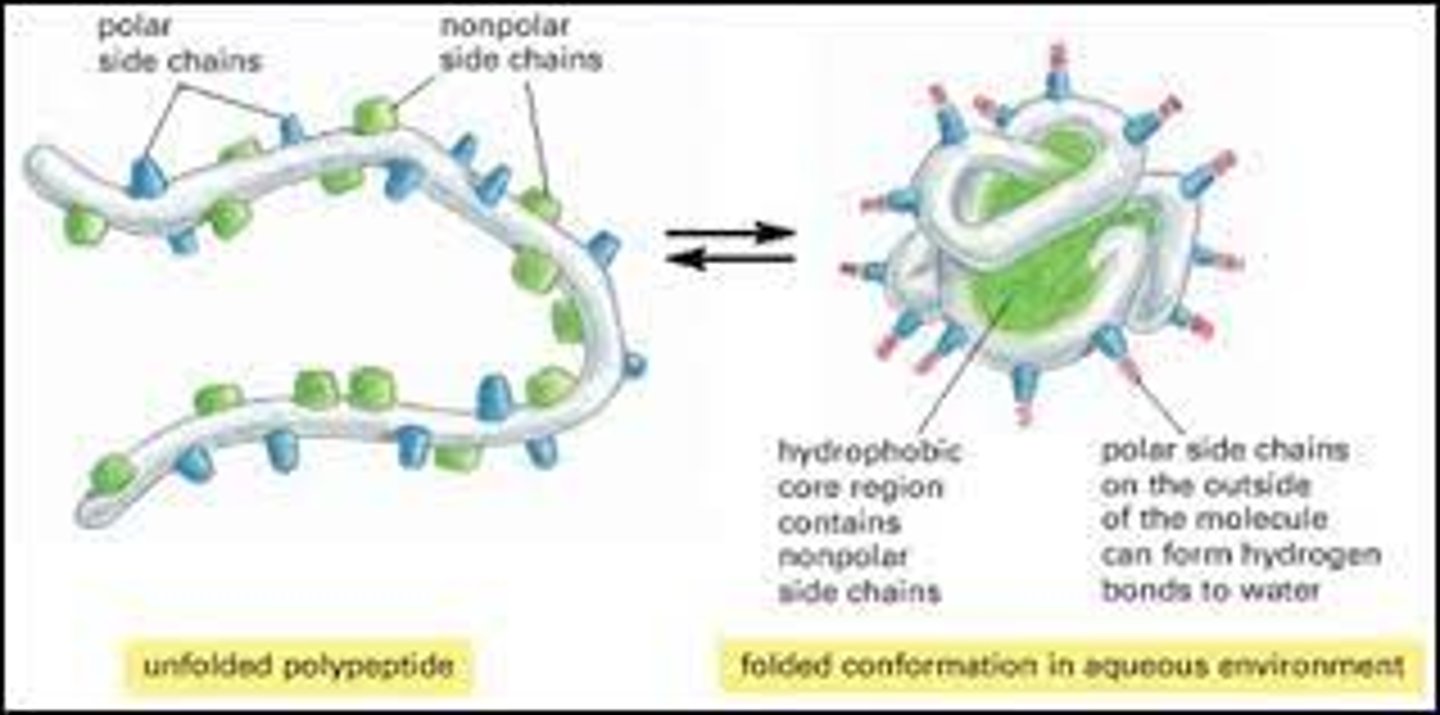
What is tertiary structure of a polypeptide? Name the bonds that are involved in theformation of this level of structure in a polypeptide.
-refers to the overall three-dimensional arrangement of its polypeptide chain in space. It is generally stabilized by outside polar hydrophilic hydrogen and ionic bond interactions, and internal hydrophobic interactions between nonpolar amino acid side chains
Where do you expect non-polar amino acids in the tertiary structure of a polypeptide and how do they interact with each other?
-polypeptide folds into its correct shape, amino acids with nonpolar side chains usually cluster at the core of the protein, staying away from water.
-van der Waals forces, also known as London-dispersion forces. These forces are attractive between non-polar side chains, which are hydrophobic and cling together in polar solvents.
Which amino acid forms the disulfide bond and how?
https://www.google.com/url?sa=i&url=https%3A%2F%2Fm.youtube.com%2Fwatch%3Fv%3DY80Ro8k4iJA&psig=AOvVaw0T19zc4KHTnxEebfgg8UU2&ust=1696284549473000&source=images&cd=vfe&opi=89978449&ved=0CBIQjhxqFwoTCLCdxsHu1YEDFQAAAAAdAAAAABAE
cysteine contains a thiol group and readily forms disulfides so linking two cysteine units together.
-Disulfide bonds are formed between cysteine residues through a process called oxidative folding. The process occurs when the polypeptide chain of a protein collapses, and cysteines brought together can form covalent linkages.
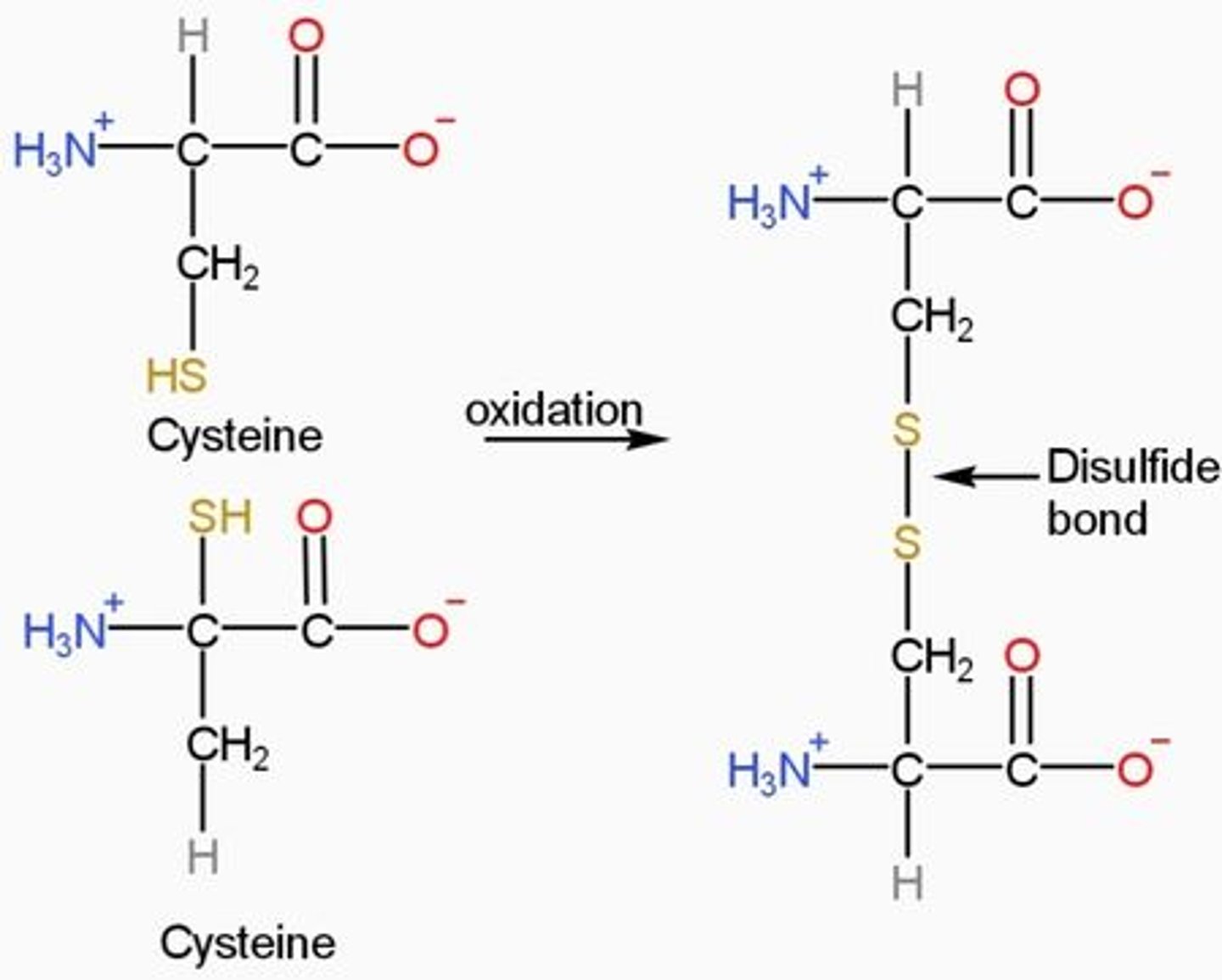
Oxidation
-Oxidation is the loss of electrons during a reaction by a molecule, atom or ion.
Oxidation occurs when the oxidation state of a molecule, atom or ion is increased.
-addition of oxygen
reduction reaction
-a chemical change in which electrons are gained, either by the removal of oxygen, the addition of hydrogen, or the addition of electrons
Be able to explain the term “quaternary structure” of a protein that is made of more than one polypeptide. Name two examples of proteins that possess a quaternary structure that we have studied so far.
Protein Quaternary Structure - an overview - ScienceDirect.com
-most intricate degree of organization of a single molecule. It describes how the subunits of a protein are arranged.
- quaternary structure is made of two or more identical of different polypeptide chain or subunits and can be arranged symmetrically.
-hemoglobin, HIV-1 protease and the insulin hexamer
-collagen
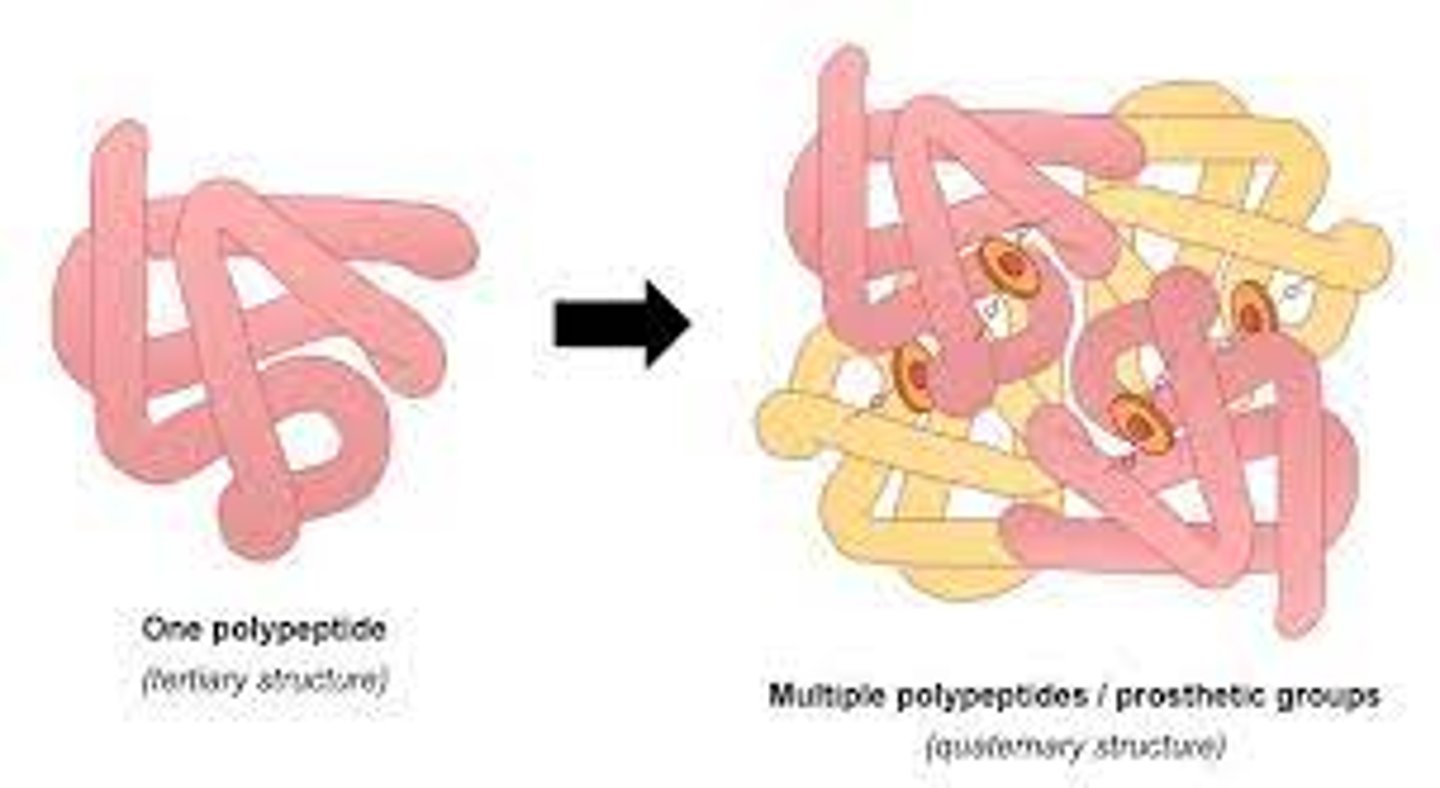
Given a sequential diagram, be able to choose which parts illustrate primary,secondary (alpha helix of beta sheet), tertiary or quaternary structures.
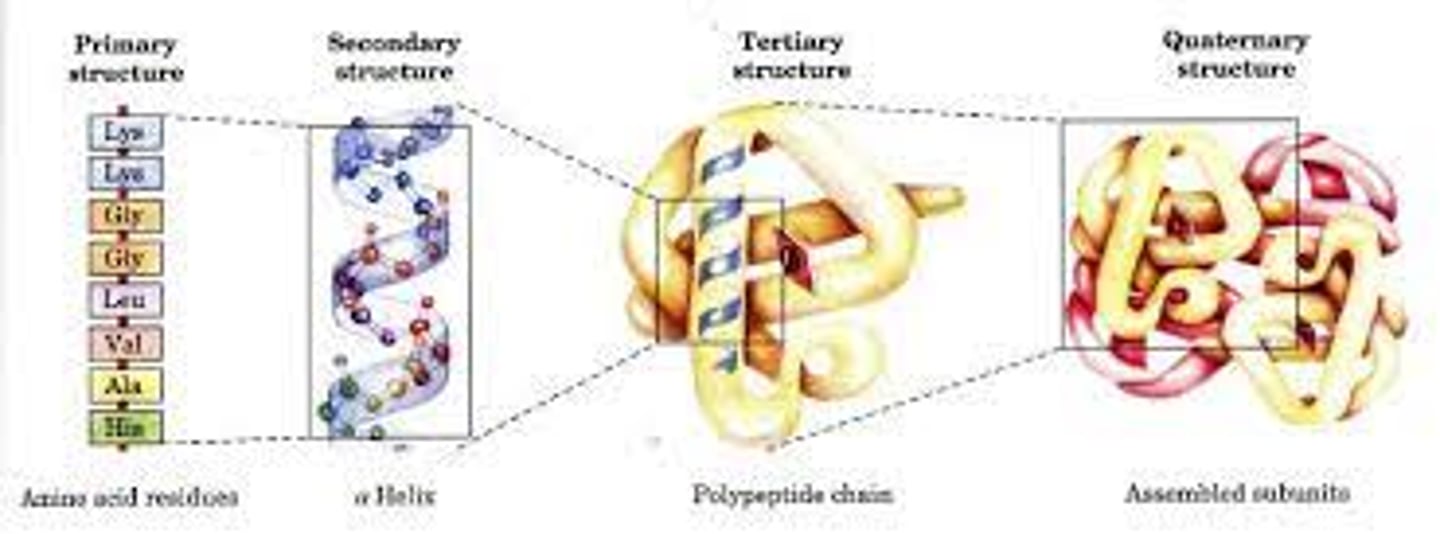
What is the most important basis for a protein to fold in a particular way that is intrinsic to it (this is a property of the protein itself)? What extrinsic factors also affect the conformation (folded structure) of a protein?
-hydrophobic packing of non-polar side chains in the core of the protein, and additional energy is provided by electrostatic interactions. The various strengths of all these interactions for a specific structure should, in theory, define its three dimensional structure.
-mainly also the sequence of amino acids
Explain what is meant by protein denaturation and renaturation.
-Denaturation leads to unfolding of the peptide chain, thus causing disorganization of the internal structure of protein. Renaturation is sometimes reversible as by placing back into the physiological conditions, the bonds can still appear and restore its original conformation.
What happens when you boil an egg? Denaturation or renaturation ? Why does it solidify?
https://www.khanacademy.org/science/biology/macromolecules/proteins-and-amino-acids/a/orders-of-protein-structure
-Egg whites contain large amounts of proteins called albumins, and the albumins normally have a specific 3D shape, thanks to bonds formed between different amino acids in the protein. Heating causes these bonds to break and exposes hydrophobic (water-hating) amino acids usually kept on the inside of the protein1,21,2start superscript, 1, comma, 2, end superscript. The hydrophobic amino acids, trying to get away from the water surrounding them in the egg white, will stick to one another, forming a protein network that gives the egg white structure while turning it white and opaque. Ta-da! Thank you, protein denaturation, for another delicious breakfast.
Why does addition of lemon juice to milk cause curdling?
-Milk contains casein. Casein groupings are spread evenly throughout the milk evenly. Casein has a tendency to get precipitated and aggregated. When lemon juice is added, it increases milk's acidity because lemon contains citric acid. When milk becomes acidic, the negative charge which keeps the casein separate is neutralized. Because of the lowering of pH, the calcium causes precipitation of casein and milk gets curdled.
1. Name the two kinds of nucleic acids?
deoxyribonucleic acid (DNA) and ribonucleic acid (RNA)
2. What are the different roles that nucleic acids participate within a cell? Explain the term polynucleotide? Which covalent bond connects the nucleotides along a single polynucleotide chain? Which of the two reactions must be involved in creating this bond, dehydration or hydrolysis?
-carry genetic information which is read in cells to make the RNA and proteins by which living things function.
-Polynucleotide's are just many monomer nucleotides bonded together to form nucleic acids.
-phosphodiester bond: It acts as the connection between two nucleotides or adjacent nucleotides in the DNA strand.
-dehydration synthesis or condensation reaction.

3. Be able to describe the flow of information during gene expression.
DNA → RNA → protein. This directional flow of information is known as the central dogma of molecular biology.
4. What does DNA and RNA stand for?
deoxyribonucleic acid (DNA) and ribonucleic acid (RNA),
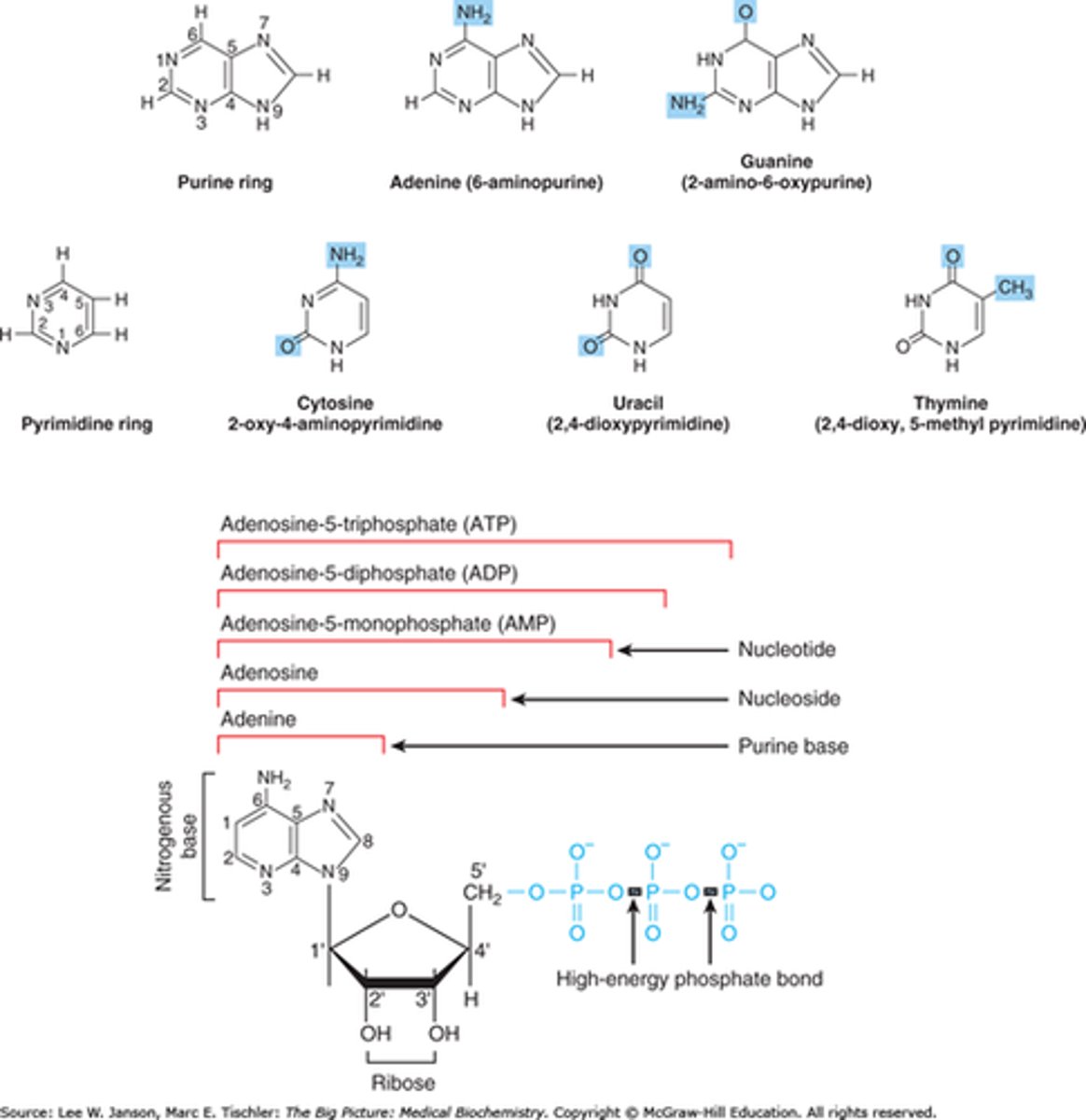
5. Name the three parts of a nucleotide.
5 carbon atoms, a phosphate group, and a nitrogenous base.
6. What is the backbone of a polynucleotide chain composed of?
The sugar and phosphate form a solid backbone to the polynucleotide chain
7. Which of the two parts of a nucleotide are together called a nucleoside?
-Nucleosides (bottom) are made of a nitrogenous base, usually either a purine or pyrimidine, and a five-carbon carbohydrate ribose. A nucleotide is simply a nucleoside with an additional phosphate group or groups (blue); polynucleotides containing the carbohydrate ribose are known as ribonucleotide or RNA.
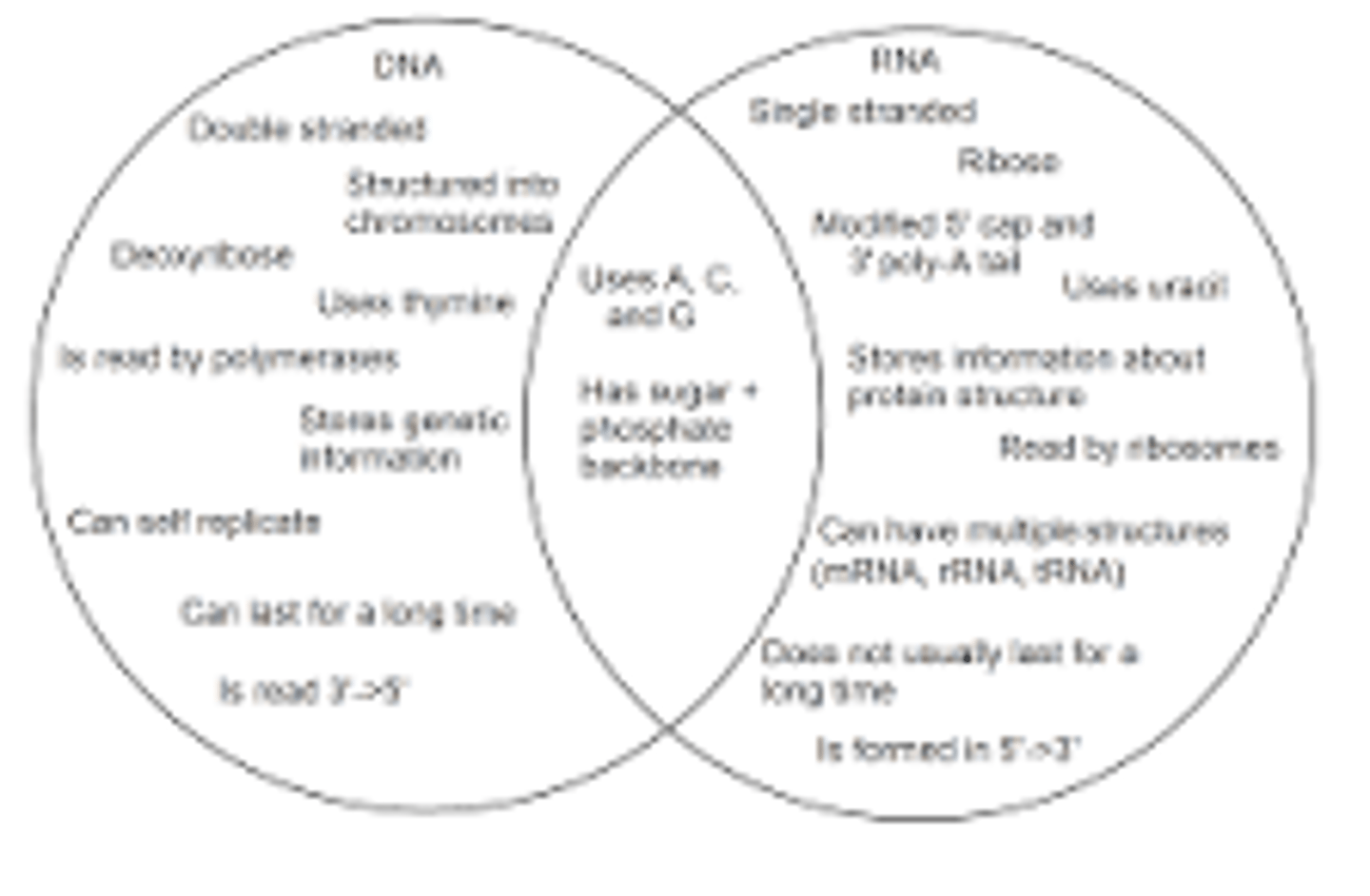
8. Be able to summarize the three distinguishing features of DNA and RNA.
RNA is single-stranded while DNA is double-stranded. RNA contains uracil while DNA contains thymine. RNA has the sugar ribose while DNA has the sugar deoxyribose.
9. Which of the three parts of a nucleotide differs only by a single atom on a carbon atom between DNA and RNA?
The sugar in DNA is 2′-deoxyribose, while the sugar in RNA is ribose. The only difference between the two sugars is that 2′-deoxyribose has one less oxygen atom attached to the second carbon.
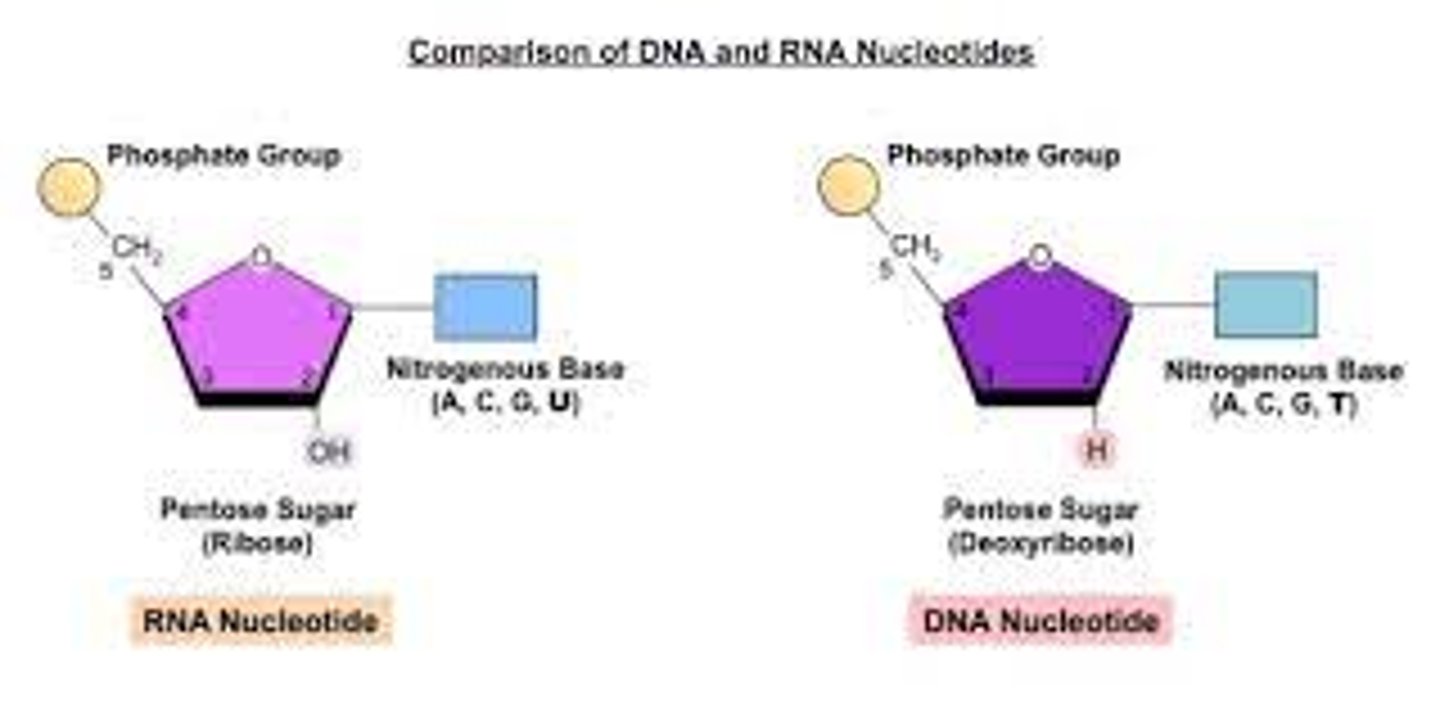
10. Given the pentose sugar ribose or deoxyribose, be able to identify whether it would be present in DNA or RNA.
The difference between the sugars is the presence of the hydroxyl group on the second carbon of the ribose and hydrogen on the second carbon of the deoxyribose (so deoxyribose is "missing" an -OH group).
11. Explain how the backbone of a polynucleotide has a very specific orientation from one end of the polynucleotide to another namely 5' to 3'.
DNA is composed of two strands that run in opposite directions, known as anti-parallel strands. The reason for this is that the DNA molecule is built using the complementary base pairing rule, where adenine (A) pairs with thymine (T) and guanine (G) pairs with cytosine (C).
If the two strands of DNA run in the same direction, the bases on one strand would not be able to bond with the bases on the other strand, making replication and transcription impossible.
DNA is always synthesized in the 5'-to-3' direction, meaning that nucleotides are added only to the 3' end of the growing strand. As shown in Figure 2, the 5'-phosphate group of the new nucleotide binds to the 3'-OH group of the last nucleotide of the growing strand.
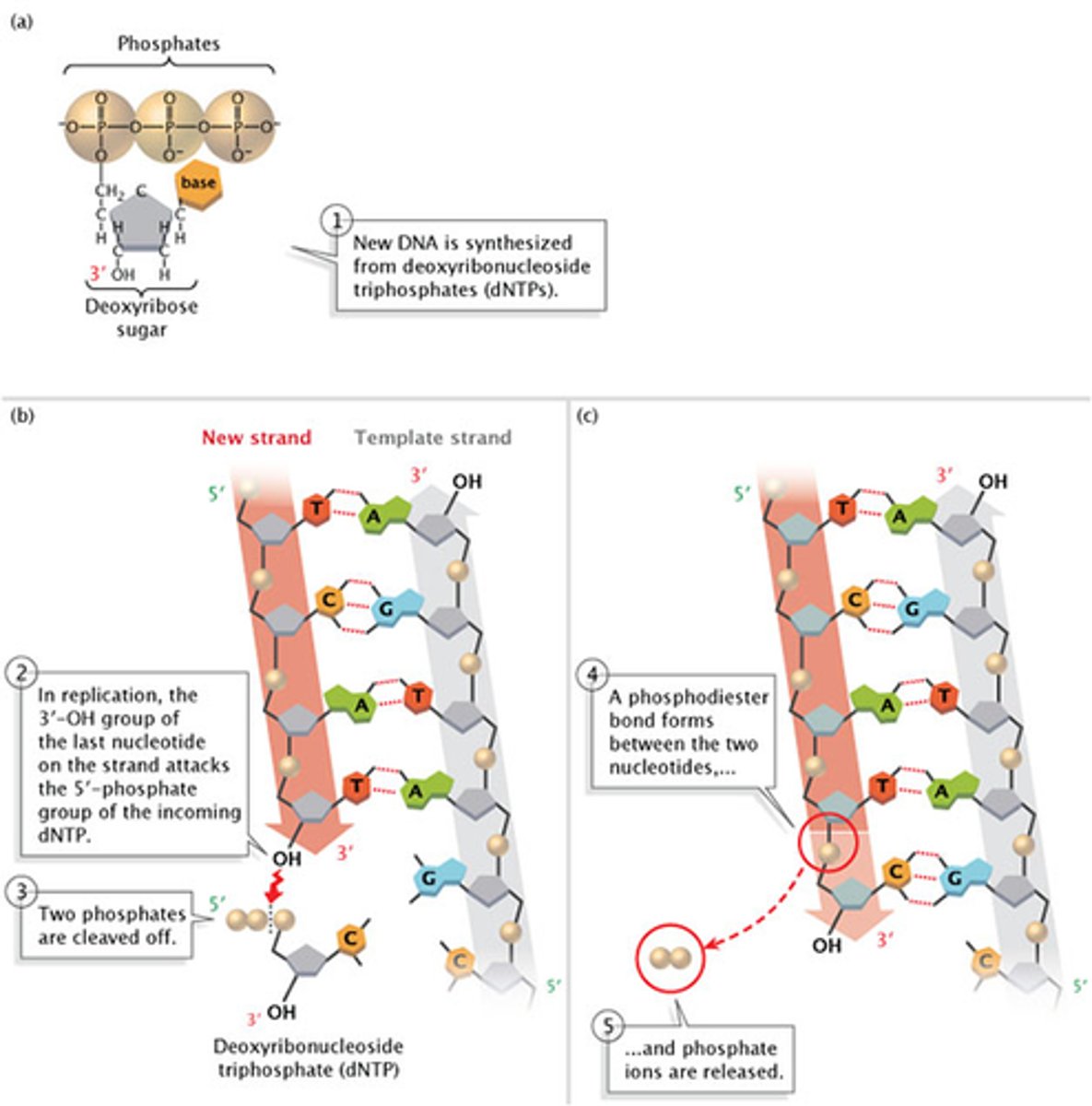
12. Given a nucleotide within a polynucleotide chain, be able to predict its 5' to 3' orientation.
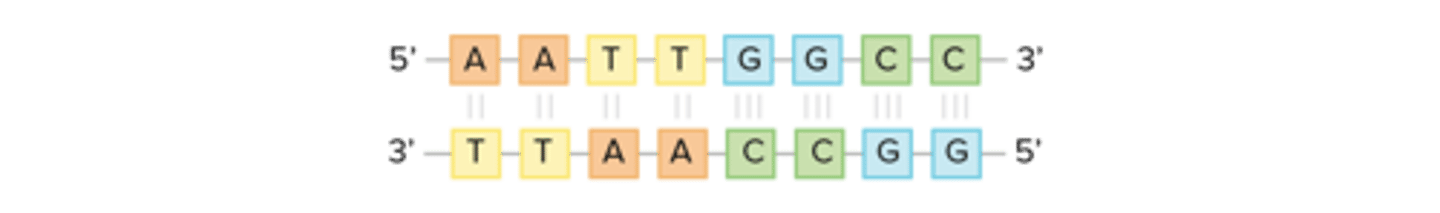
-DNA is only synthesized in the 5' to 3' direction. You can determine the sequence of a complementary strand if you are given the sequence of the template strand.
-For instance, if you know that the sequence of one strand is 5'-AATTGGCC-3', the complementary strand must have the sequence 3'-TTAACCGG-5'. This allows each base to match up with its partner:
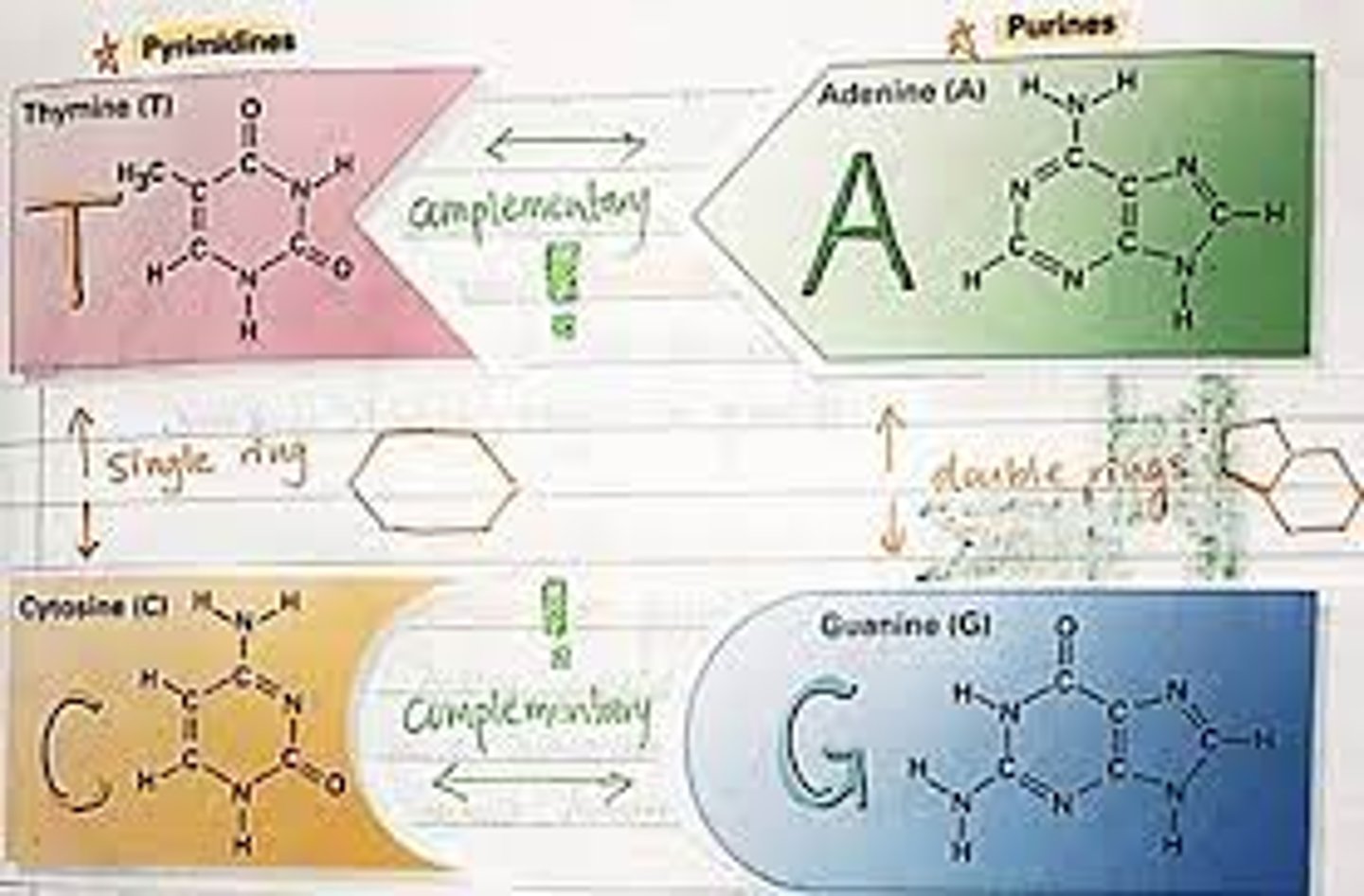
13. Name the nitrogenous bases that are present in DNA and in RNA. Be able to classify them in pyrimidines and purines. Which have single rings and which contain double rings?
-The purines in DNA are adenine and guanine, the same as in RNA.
-The pyrimidines in DNA are cytosine and thymine; in RNA, they are cytosine and uracil.
-Purines are larger than pyrimidines because they have a two-ring structure while pyrimidines only have a single ring.
14. The alternate choices of the four bases at a particular nucleotide position within a single strand of polynucleotide and the 5' to 3' orientation of the polynucleotide allows living systems to store information within this sequence from one end to another (just like the alphabets that form words). Be able to explain how DNA uses the above property to store genetic information.
genetic info is stored in DNA from specific sequences of the nitrogenous bases. (G, A, C, & T).
Bases from genetic codes in the form of triplet codons i.e sequence of 3 bases represents a particular amino acid
15. Describe the DNA double helix. What is the orientation of the two polynucleotide strands with respect to one another. How does a nitrogenous base on one strand pair on the nitrogenous base of the other strand (which bond)? Be able to explain the A-T base pairing and the G-C base pairing seen between the two antiparallel polynucleotide chains or strands of DNA molecules. Be able to explain why this is useful in replicating DNA.
-DNA molecules have an antiparallel structure - that is, the two strands of the helix run in opposite directions of one another. Each strand has a 5' end and a 3' end.
-The two strands are held together by hydrogen bonds between pairs of bases: adenine pairs with thymine, and cytosine pairs with guanine.
-A and T are found opposite to each other on the two strands of the helix, and their functional groups form two hydrogen bonds that hold the strands together. Similarly, G and C are found opposite to each other on the two strands, and their functional groups form three hydrogen bonds that hold the strands together.
-Structure: hydrogen bonds between nitrogen bases are critical to stabilize DNA double helix structure.
Complementarity: Watson-Crick base pairing (A-G, C-T) allows complementarity between DNA double strands. This is vital in replication processes, when each DNA strand serves as a template for the synthesis of a new strand.
Upvote9
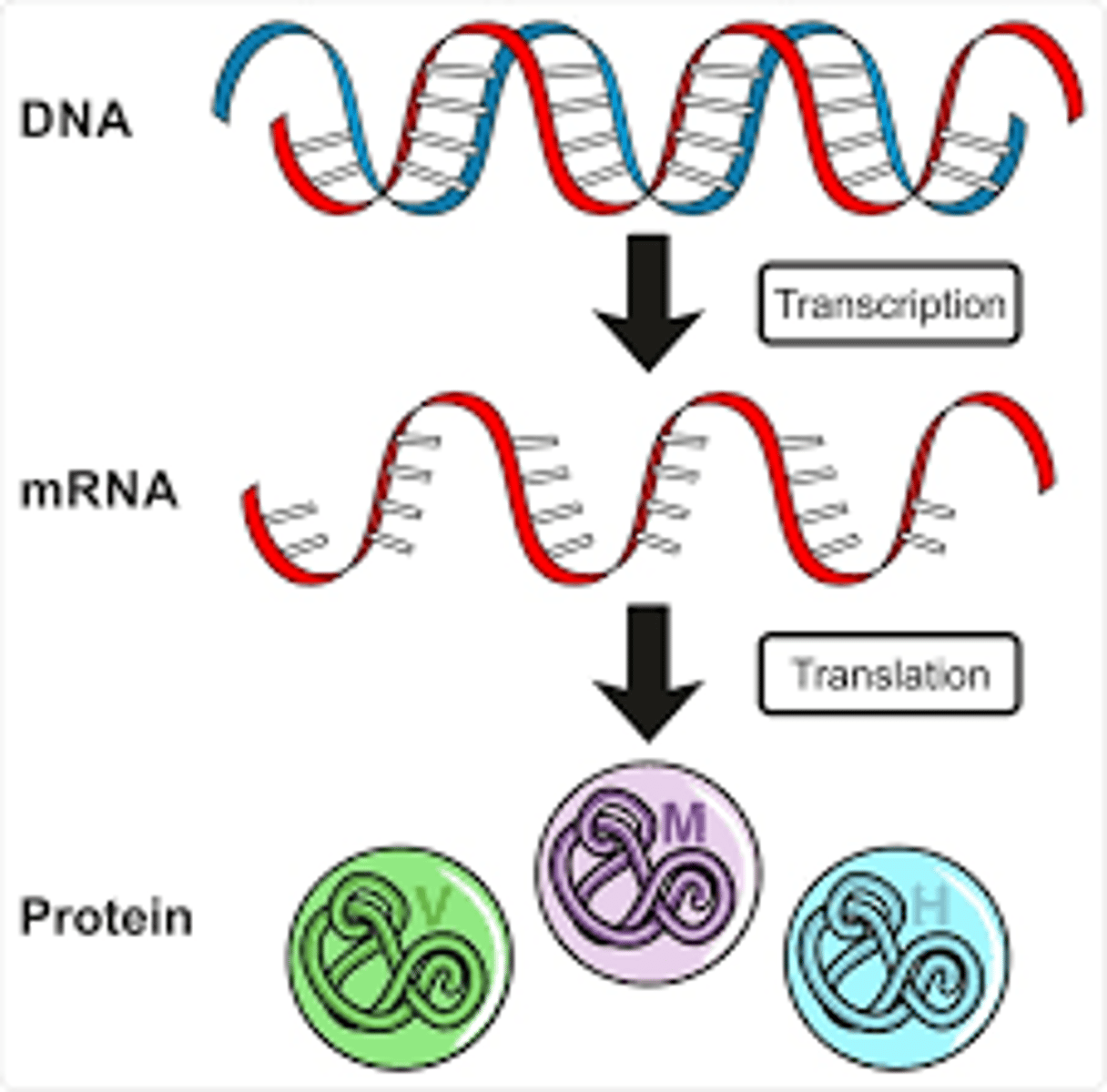
16. Explain Gene expression
-consists of two major steps: transcription and translation
-the process by which the information encoded in a gene is turned into a function. This mostly occurs via the transcription of RNA molecules that code for proteins or non-coding RNA molecules that serve other functions.
-Gene expression is the process by which information from a gene is used to produce a functional gene product. This process is tightly regulated and allows a cell to respond to its changing environment.
Purines and pyrimidines
-are the nitrogen bases that hold DNA strands
-Purines (double ring): Adenine and guanine
-Pyrimidines (single ring): Cytosine and thymine (uracil in RNA).
Why does DNA have two strands and RNA has one?
RNA has a hydroxyl group in its ribose backbone at the second carbon, which prevents it from forming two strands because it exerts reactivity. Whereas DNA has two strands because it lacks the hydroxyl group and has a hydrogen atom on the second carbon, making it more stable and less reactive.
What are the differences between alpha 1 4 and beta 1 4 glycosidic linkage?
There are are two types of glycosidic bonds - 1,4 alpha and 1,4 beta glycosidic bonds. 1,4 alpha glycosidic bonds are formed when the OH on the carbon-1 is below the glucose ring; while 1,4 beta glycosidic bonds are formed when the OH is above the plane
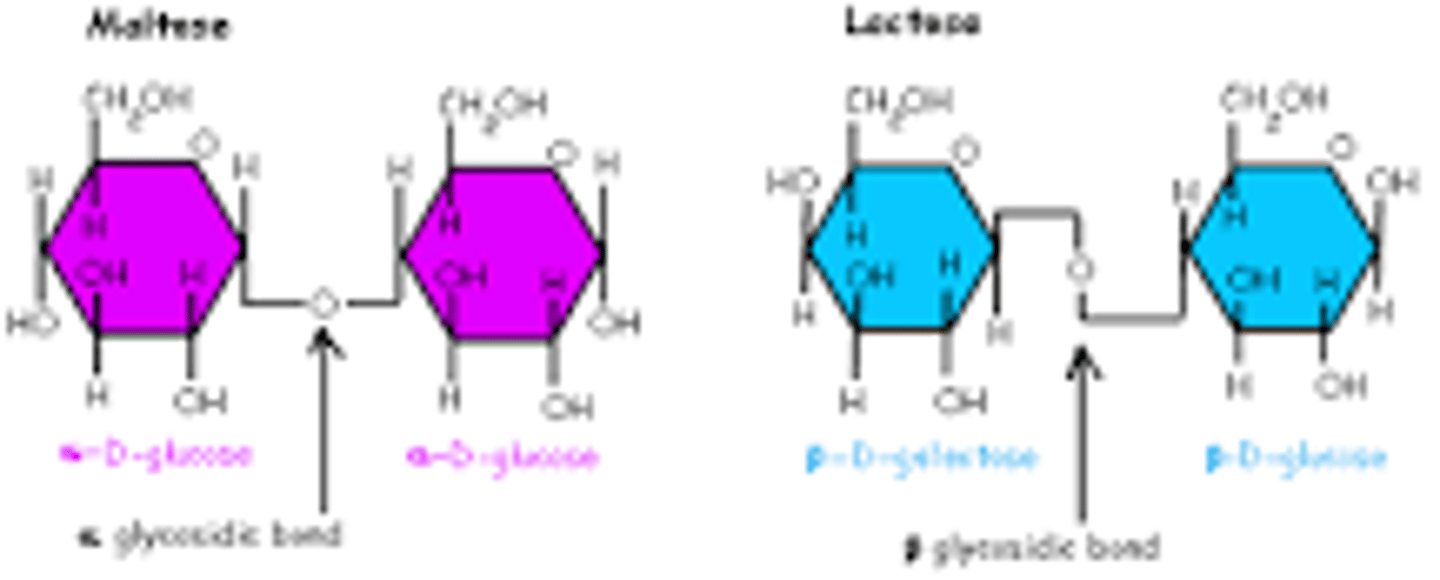
Difference between alpha and beta glycosidic linkage
alpha are when the hydroxyl groups if first glucose monomer are below the carbon 1 spot.
beta are when the first glucose monomer hydroxyl group is about the carbon 1 spot.
Beta only occur in celluose which is why humans cannot digest it.
https://www.youtube.com/watch?v=XwBE47fwPoM
why exactly are lipids not considered macromolecules.
Simply put, lipids are a type of fat having a glycerol molecule and a two or three fatty acid chain. Although there is always one glycerol, the number of fatty acids varies. As a result, there isn't a monomer unit. A polymer also has no natural set size, thus it must be terminated by a process in living organisms.
Explaining alpha and beta bonds again.
. What it doesn't say is that the alpha-glucose can convert to beta-glucose and back again. Once the linkage is formed, it cannot change from alpha to beta.
Alpha linkage has the oxygen (on the aldehyde or ketone) below the ring and the beta has it above the ring.
In beta you can't form a glycosidic bond; you have to remove a water molecule from the β�-glucose to make a glycosidic bond which is (0). so drawn out alpha and beta the −OH−OH in alpha on carbon one points below and on the adjacent carbon points below.
So water can be removed and leave behind an O hence the downward linkage you can't do that on beta so we say the adjacent beta molecule is rotated 180 degrees to get the 1-4 linkage