reaction profiles
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
What is a reaction profile?
A diagram showing how the energy of reactants and products changes during a reaction.
What does a reaction profile show?
Reaction profile is a graph that shows how the energy in a reaction changes as the reaction progresses. The graph starts at the energy level of the reactants and finishes at the energy level of the products. These two points are usually joined by a smooth curve.
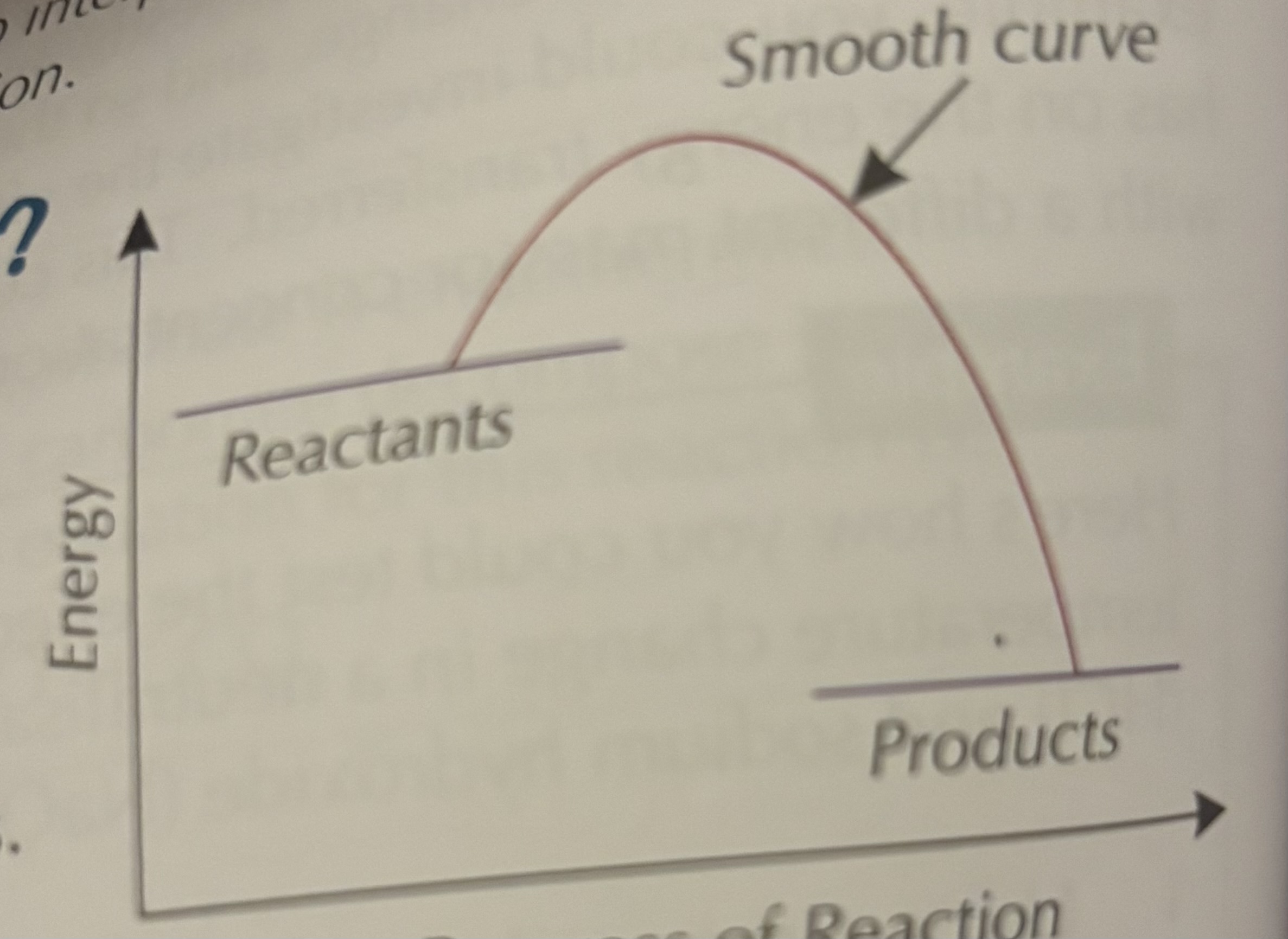
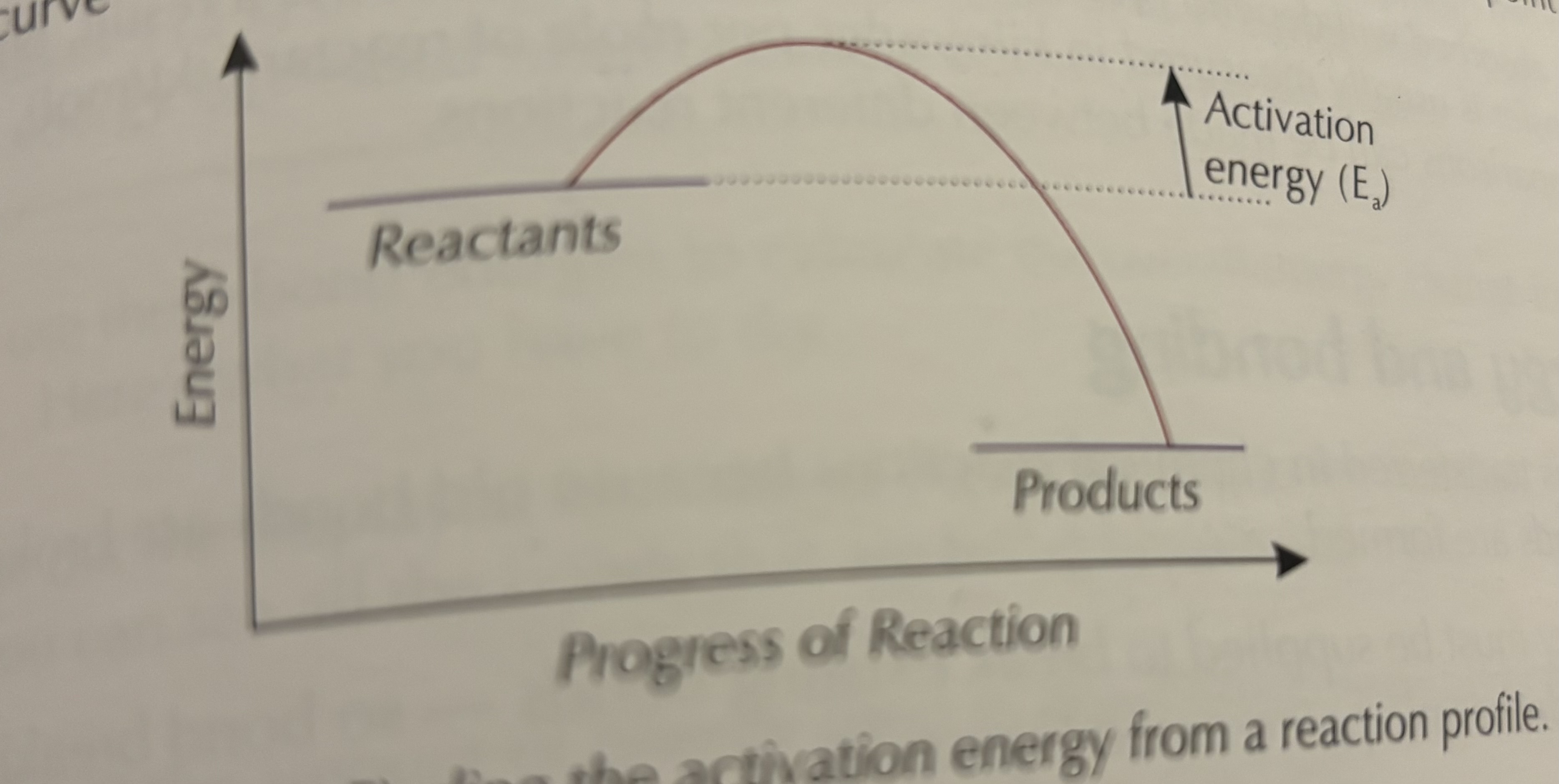
What is activation energy?
The minimum energy needed for a reaction to occur.
The greater the activation energy the more energy that is needed to start the reaction
How is activation energy shown on a reaction profile?
As the peak between the reactants and products.
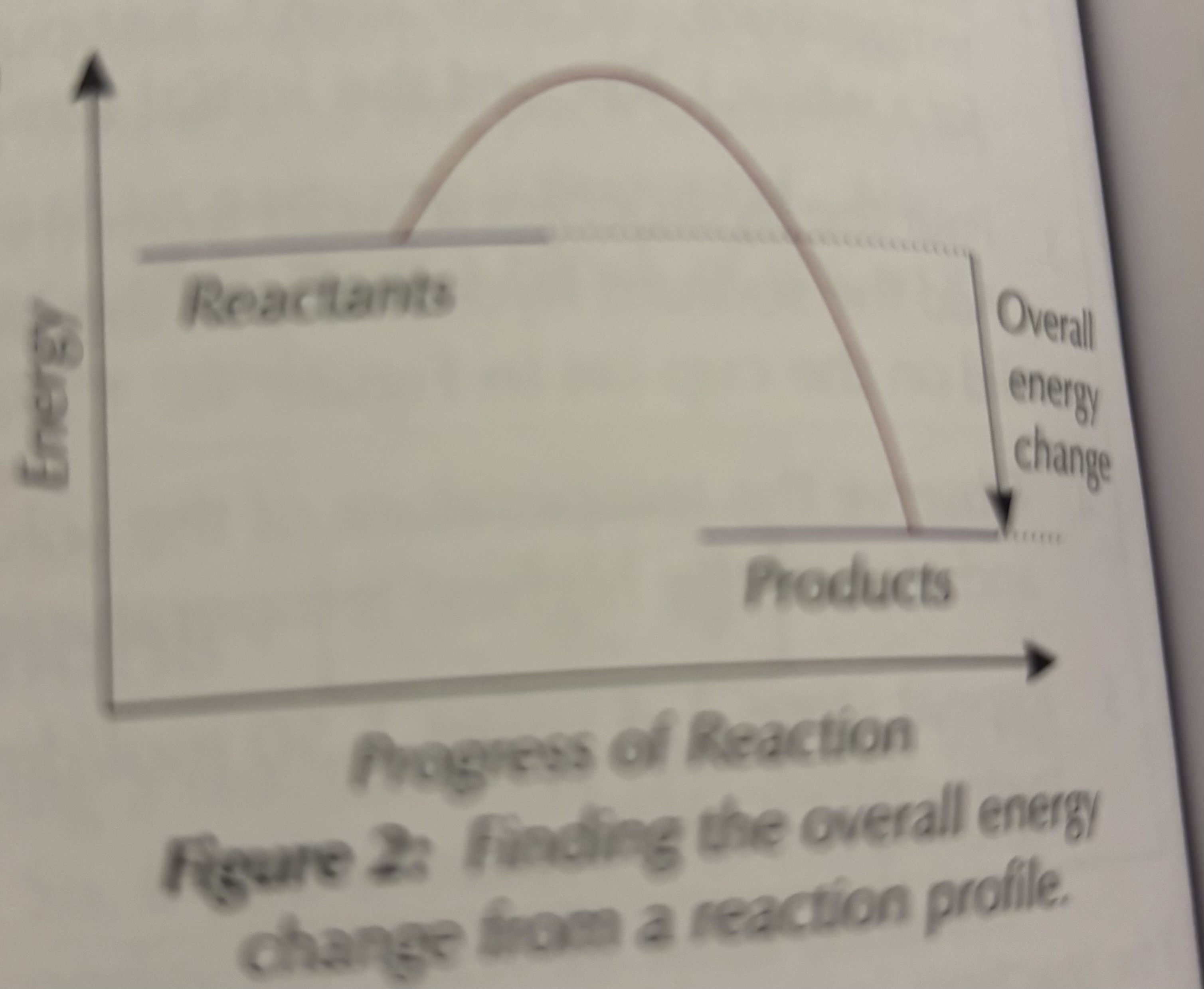
What does the overall energy change represent?
The difference in energy between reactants and products.
How do you identify an exothermic reaction from a reaction profile?
Products are at a lower energy level than reactants.
How do you identify an endothermic reaction from a reaction profile?
Products are at a higher energy level than reactants.
What does a downward curve in a reaction profile mean?
Exothermic — energy is released to the surroundings.
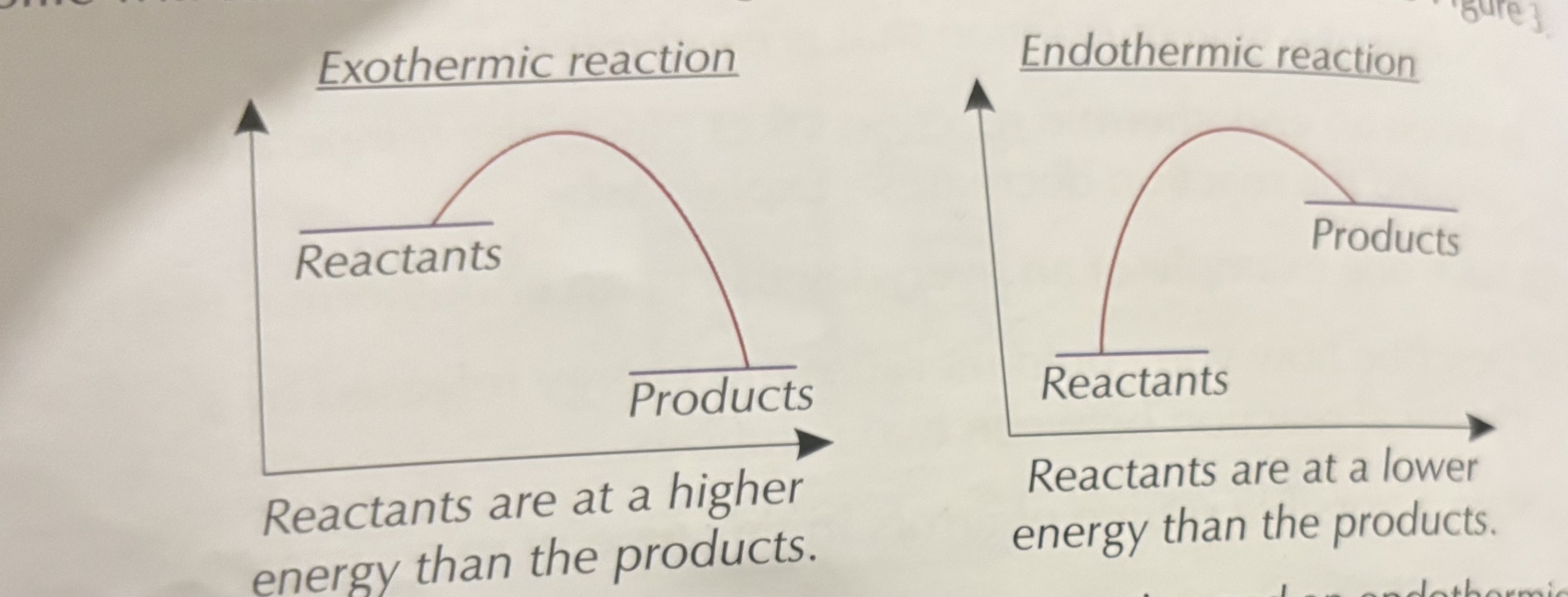
What does an upward curve in a reaction profile mean?
Endothermic — energy is absorbed from the surroundings
Why do some reactions need a lot of activation energy?
To break bonds in the reactants and start the reaction