intro and pharmacokinetics
1/252
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
253 Terms
pharmacokinetics simple definition
drug absorption, distribution, metabolism, elimination. what the body does to the drug, factors controlling drug conc in body or at target site, relationship bw dose and time (time course)
pharmacodynamics simple
drug targets, drug receptor targets and second messengers, efficacy, potency, drug response relationships
toxicology simple
adverse drug responses, therapeutic window, toxicity testing
drug development simple
discovery, pre-clinical studies, clinical studies, post-marketing surveillance, herbs as drugs, over the counter medication
pharmacology definitions
study of drugs: how they react biologically at receptor sites in body. drugs effects: ehat it does once it is taken by someone, esp as part of a medical treatment. branch of medicine that deals w actions of drugs in the body (therapeutic and toxic), describes dvlpmnt and testing of new drugs, and explores the uses of existing drugs
types of pharm studies
basic science (fate and action of drug, molecular to body, and in any animal). clinical applied science (fate and action of drug, treatment of disease, primarily in humans but also vet and wildlife). what is happening inside a diseased person is different for a non-diseased person
history of pharmacology
independent recognition at end of 19th cent in GER. use of drugs much longer (plant based) → cinchona bark (malaria, quinines), foxglove (cardiac disease, digoxin), etc. synthetic orgo started in 1828
Kalant statement on what a drug is
not completely applicable anymore as insluin, antibodies, etc are made by the body but are also given as medication

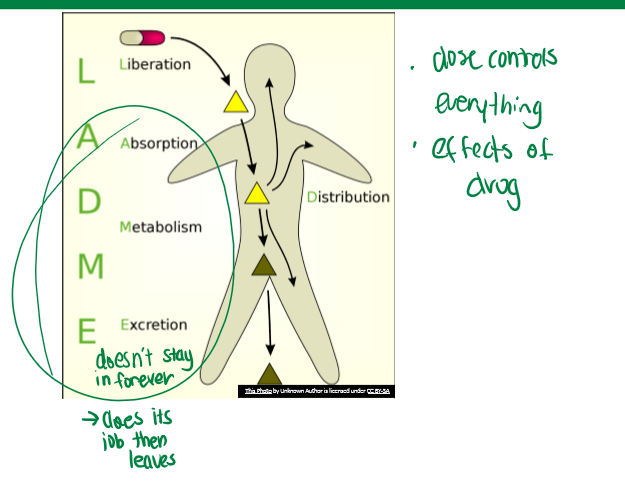
LADME
pharmacokinetics. Liberation, Absorption, Distribution, Metabolism, and Excretion
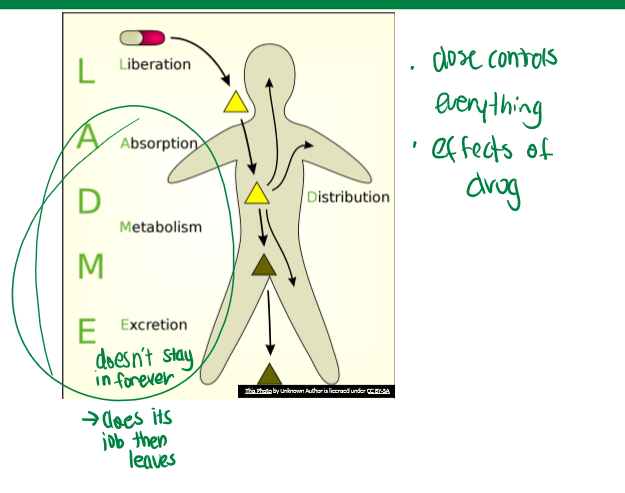
drug liberation
not critical for every drug. time of it depends on encapsulation material (hard is gelatin, glycerin, sugar, and water; soft is gelatin, glycerin, and water). will impact dosage in body. how drug is formulated and brought to the taker
drug absorption overview
movement of drug into body, typically into blood, must pass barrier (typically epithelium), sites depend on drug delivery system. the process by which drugs enter the body or the cell
common sites of drug absorption
IV, respiratory tract, skin, sublingual, oral, rectal, eye, ear, etc. all must get across some epithelium in some way
drug distribution overview
most absorbed drugs enter blood compartment, utilize blood flow/circulation to distribute drug to specific tissue or site of action. some factors affecting this include: flow, tissue barriers (BBB), proteins in blood (whether carrier or lipophilic), etc.
drug metabolism overview
since drugs are xenobiotic, there are extensive systems for their metabolism like large enzyme families. biotransformation is key for this. this is important so that it does its job but does not stay around as an active compound forever. the major organ responsible is the liver
drug excretion overview
removes drug or drug metabolite from body to prevent accumulation of xenobiotics. major organs for removal are: intestines (lipophilic, feces), and kidney (soluble, urine)
dose
amount that enters the body or cell. mg or mg/kg; ml (liquid). amount given at a specific time
dosage
includes schedule of administration; considers frequency of administration, number of doses over time
dosage in latin
many things in pharm and dosages are in latin. po= per os (oral meds); bid/bis/di=twice a day; prn=prove nata (as needed);
why there are different routes of administration
changes absorption and factors that affect it. things outside of oral administration would be used for a drug that may get destroyed in stomach acid. IV is used when time is of the essence (also means it cannot be removed as soon as administered), also for continuous administration (when very ill).
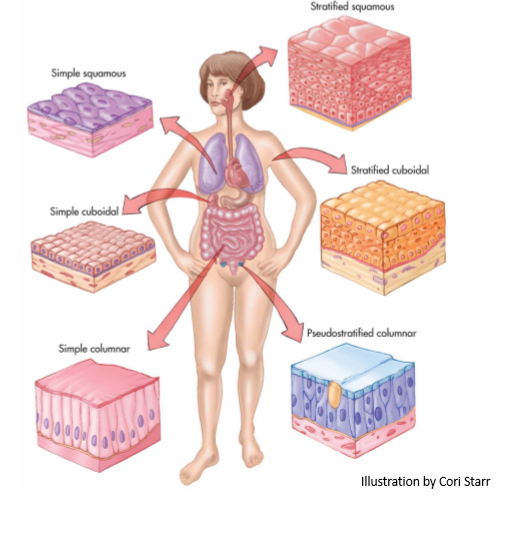
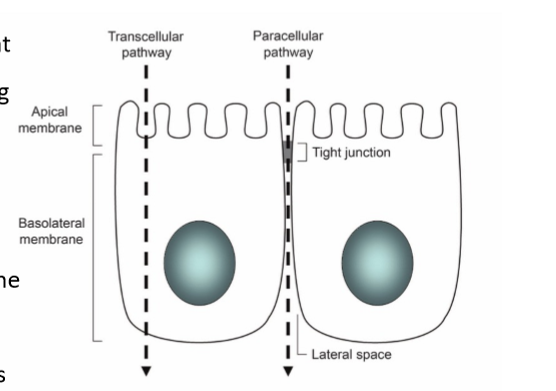
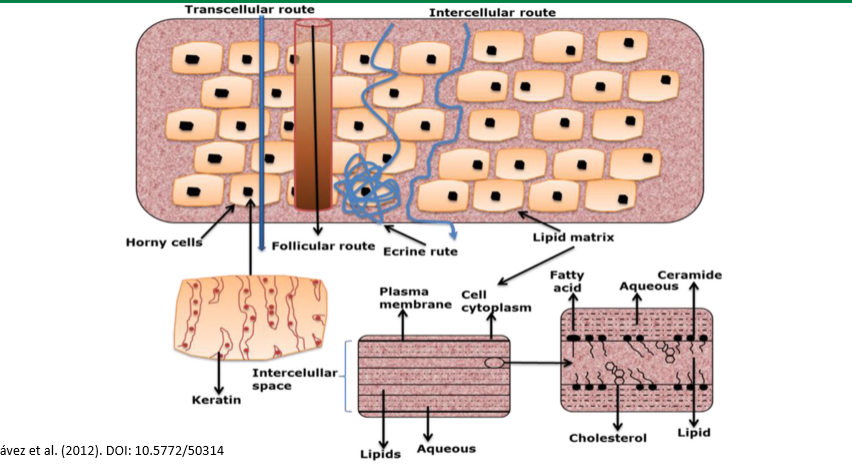
epithelium
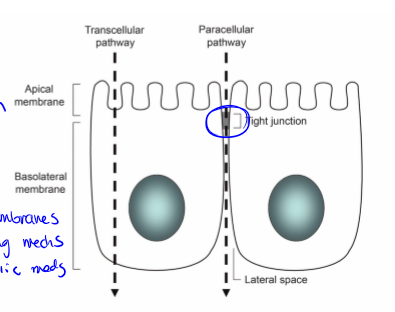
gatekeeper to what gets in the body. has high selectivity. the primary barrier to absorption. to exert their effects, drugs must first pass through the cell membrane through paracellular or transcellular transport
paracellular transport
transport between cells. passive process, route controlled by tight junctions: presence of occludins, claudins, and junction adhesion molecules. this is the typical route for hydrophilic drugs since they can’t pass through the phospholipid membrane alone
transcellular transport
transport across/through cells. lipids bilayer is semipermeable barrier, protein and lipid content varies, molecules may pass the lipid bilayer by: diffusion, transporters, and vesicle mediated. problem: two membranes, different passing mechanisms, usually lipophilic drugs. can be active or passive
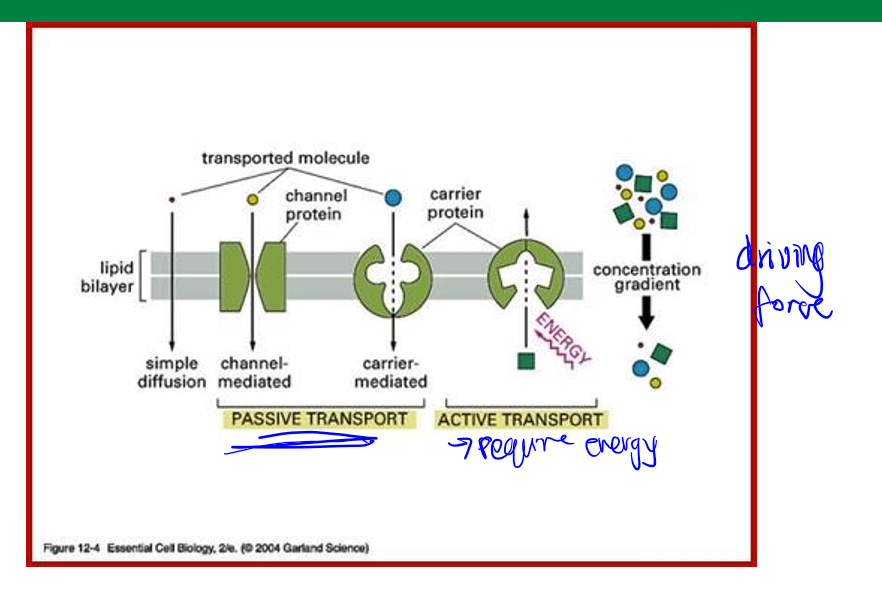
diffusion
passive transcellular transport. molecules pass by diffusion depending on: lipophilicity (best is lipophilic), charge (uncharged), size (less than 400 daltons).
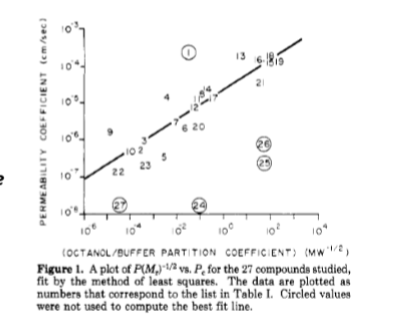
lipid-water coefficient
Log Kow (organic water). partitioning coefficient good indication for diffusion. k=Co/Cw where Co is the total concentration in organic phase (typically octanol) and Cw is the total concentration in water/aq phase. larger K means larger permeability. (circled numbers are those charge or size limited as lipophilicity is not the only distinction)
transport mediated transcellular transport
protein mediated, facilitated diffusion (passive, through protein channels and carriers), active transport (energy-consuming proteins), and ATP binding cassette (ABC) superfamily of transporters
vesicle mediated transcellular transport
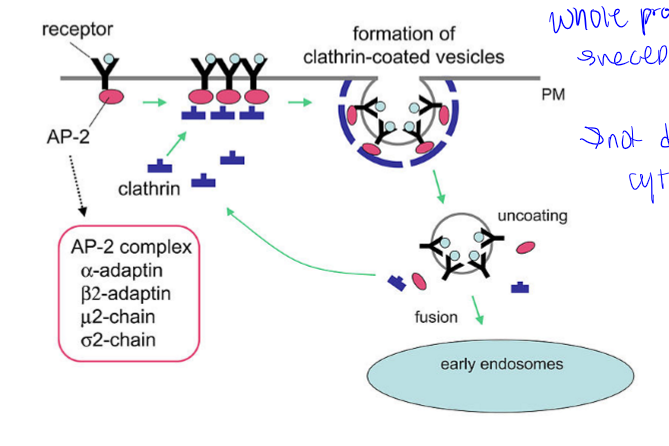
endocytosis= pinocytosis (fluid and solids) and phagocytosis (particle uptake). major route through clathrin-dependent processes
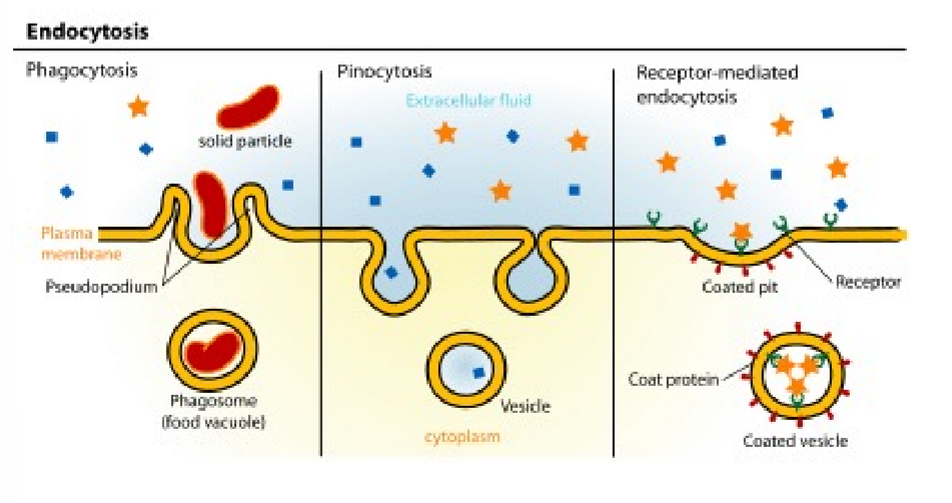
clathrin-dependent endocytosis
whole process, receptor mediated, not directly into cytoplasm
mucosal membranes
site of absorption. layer of epithelial tissues which lines areas of body that come into contact w air. this is the digestive tract (mouth, stomach, intestine, and rectum) and respiratory tract (nasal cavities, airways, lungs)
major drug barriers
epithelial cells often polarized, different properties in the cell membrane depending on orientation. there is apical, basolateral, gut barrier, and bbb
apical membrane
facing the lumen or external environment
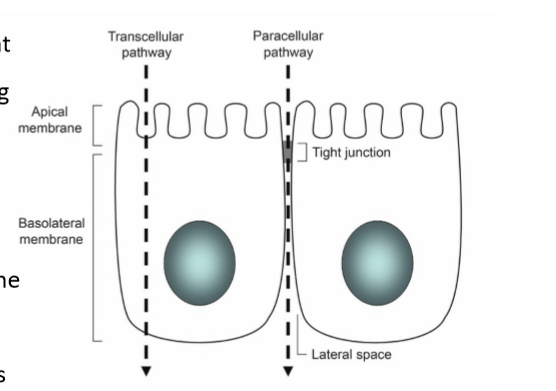
basolateral membrane
facing the basement membrane
gur barrier
intestinal epithelial cells
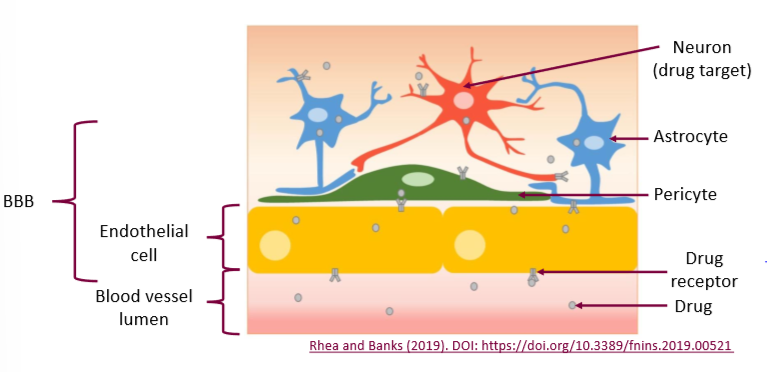
bbb
blood brain barrier endothelial cells. very specific proteins to limit or increase transport. major issue for pharmaceuticals
things affecting route of administration
(roa determines barrier), cultural background, compound, condition/disease, patient compliance (patient doing what physician asks them to or not)
enteral RoA
thorugh GI tract, oral is an example. A: convenient, cheap, good absorption. D: risk of not working is high, may irritate gastric mucosa, unpleasant taste, cannot take if unconscious, babies or elderly cannot take, affected by food intake
parenteral RoA
injection of some sort, in solution, other injections include: intramuscular, subcutaneous, intraspinal, intraperitoneal. A: rapid, can be used unconscious, avoids gastric irritation. D: painful, expensive, risk of infection, administrator needs training.
topical RoA
tough definition, usually means local effects but not always. examples are eye, ear, nose, skin, lung (sometimes). absorptive barrier (resp mucosa or skin epidermis), may be absorbed into mucosa/skin alone or through to deeper layers including plasma. variation in absorption with: drug lipophilicity, SA for application, vehicle/base, hydration and thickness of skin. A: high local conc, low systemic effect. D: low onset, local reaction, limited drugs, systemic effects may lead to local tissue destruction
intravenous RoA pros
directly into circulation, no absorption barrier (faster, higher than any other RoA, only case where administered dose=absorbed dose bc sln directly into plasma.
intravenous RoA cons
no removal possible after injection, must be sterile, possible infection risk, training needed to administer, painful, compliance issues (cultural background, severity of disease/situation), dose decreases over time if not continuously administered (IV drip)
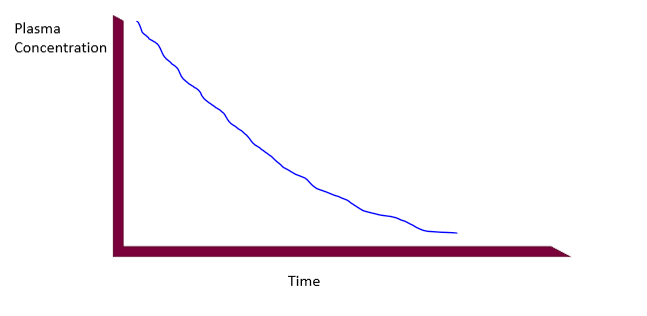
IV absorption curve (bolus)
immediately delivered to plasma, needle breaches barrier, 100% absorption, plasma concentration in highest point immediately after injection, concentration decreases with time
epidermis
stratum corneum, stratum lucidum and granulosum, stratum spinosum, and stratus germinativum
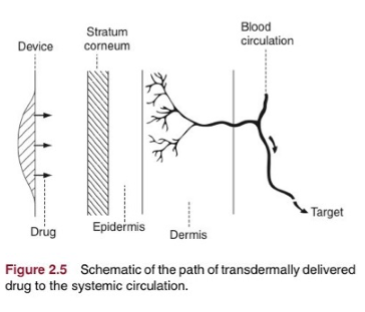
stratum corneum
top layer of epidermis, dead keratinocytes pressed together, horny layer
stratum lucidum and granulosum
second epidermic layers from top, thinnest layers, cells flatten and adhere and die, pushing up to corneum
stratum spinosum
third epidermic layer from the top, thickest later, keratinocytes
stratum germinativum
lowest layer of epidermis. basal cell layer, stem cells, constantly regenerating, pushing up cells
dermis
next layer after epidermis. blood, lymph vessels, hair follicles, etc. high in collagen and elastin. this is where the drug wants to get to
subcutaneous layer
lowest layer of skin under dermis. fatty layer
types of patches
fentanyl, morphine, steroids (birth control, HRT), nicotine (most common). this is where we question whether topical really means local since these all affect things outside the local site
transdermal RoA
drug delivery to circulation through skin (not topical), may include ointments, creams, and patches. nicotine patch is slow release, great for stable concentration release. less variable concentration in circulation. increase compliance by reducing re-medication (issue is obvious), important factors are: molecular size, kow, mp, potency, half-life, absorption must be reasonable over small SA, placement on body matters
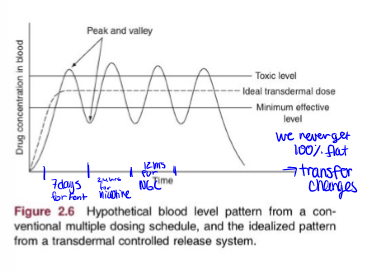
transdermal route absorption curve
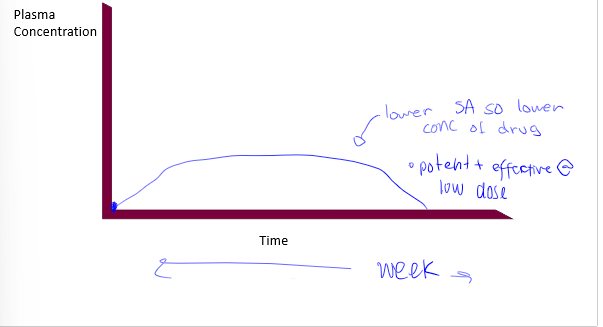
ideal transdermal drug delivery
with continuous redosage thorugh patch
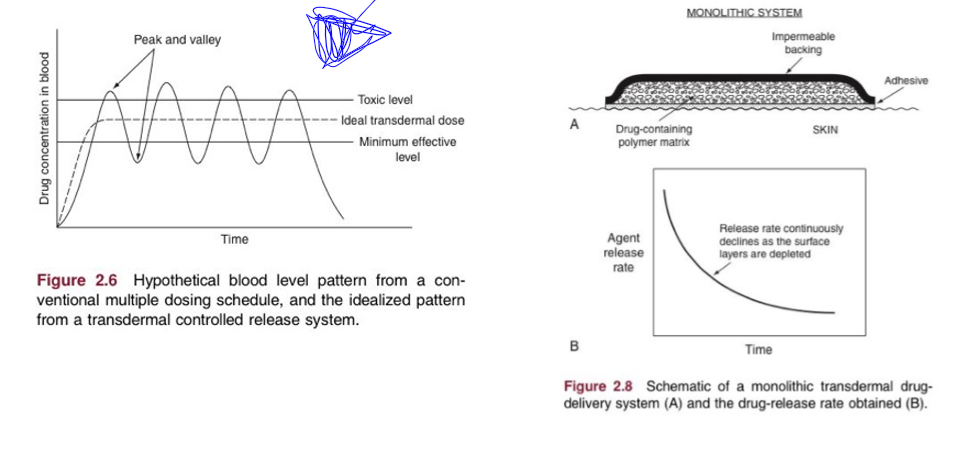
transdermal patch issues
compound size, lipophilicity, dose required, increase skin permeability, increase driving force for transport. so, most frequent drugs are small, lipophilic, low dose drugs since SA is small which limits absorption
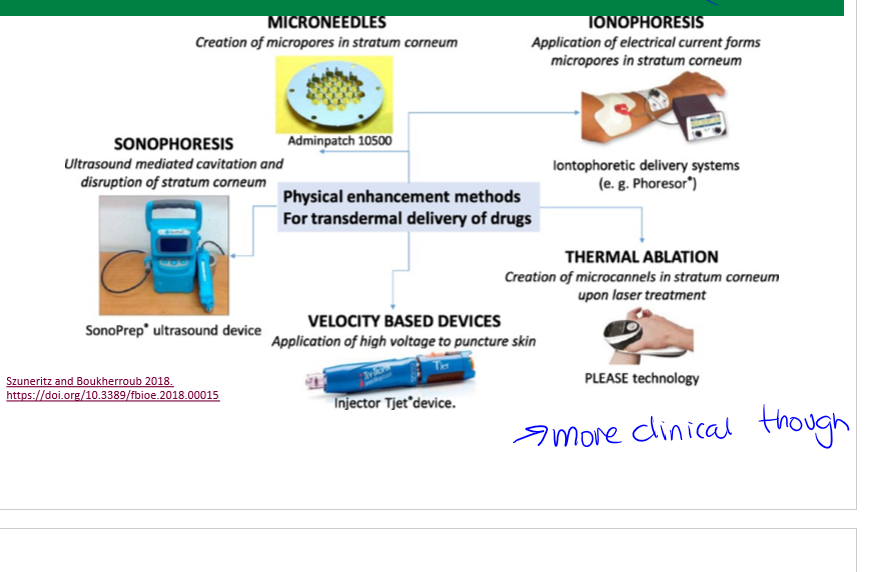
transdermal patch delivery systems
chemical enhancers, skin ablation etc to increase uptake
chemical enhancers
transdermal drug delivery systems. increase skin permeability by reversibly disrupting stratum corneum structure, provide added driving force, avoid injury to deeper tissues
skin ablations
transdermal drug delivery system enhancement, increasing absorption through pores.
oral route of administration
enteral, most frequent RoA, absorption barrier is epithelia in GI tract, easiest compliant bc convenient and cheap, safe bc time to intervene if too much drug, slower absorbance, administered and absorbed dose can be very different, huge absorption. challenges: stomach acid (harsh, degradation, lose portion of drug), metabolism by liver (higher in this route before hitting circulatory system).
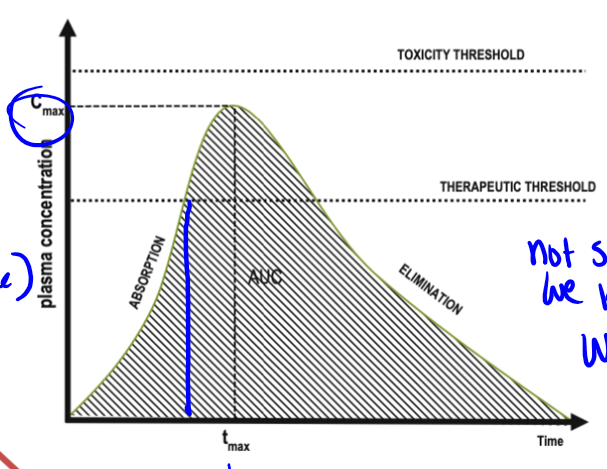
pharmacokinetic curves
plasma conc on Y axis, time on X axis. shape of curve varies with RoA, redosing scheme (if any). different processes control different parts of the curve, absorption dictating the beginning. contains minimum effective dose
minimum effective dose
the plasma dose needed for the drug to have a therapeutic effect (a sort of threshold), there is therapeutic failure if below this point, and the time spent above this provides the time for the therapeutic effect. rise enough past this to start the effect
trade off in dose
too low= therapeutic failure; too high= increased risk of toxicity; goal is to achieve a dose that maximizes therapeutic effect (dose and time) without necessarily going higher
redosing
offers flexibility to: maintain dose above min therapeutic dose, minimize dose above toxic level, and timing of redosing is dependent on other PK processes (not just absorption)
single oral dose pharmacokinetic curve
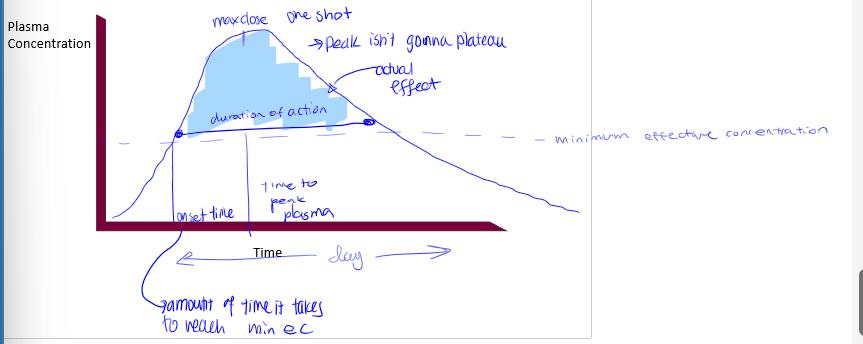
onset time
time it takes to reach minimum effective concentration
max dose
peak in pharmacokinetic curves
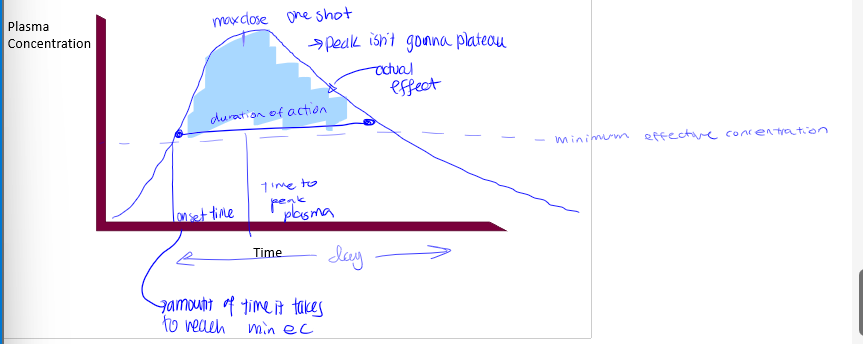
duration of action
time of actual effect
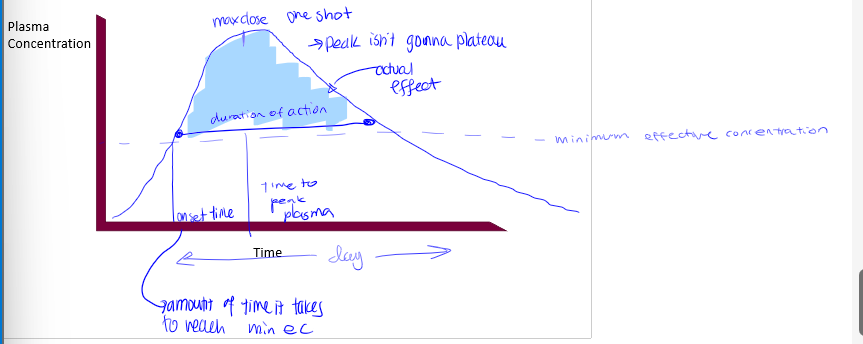
tmax
time it takes to reach peak in pharmacokinetic curve. not something we know from curve, we apply this to the curve
what processes dictate PK curves up to peak
liberation/absorption
what processes dictate PK curves after peak
metabolism will dominate and elimination may dominate if drug is not metabolized
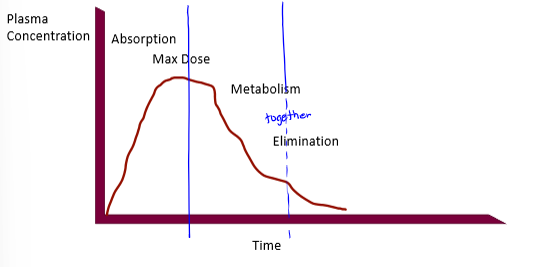
oral route absorption curve with redosing
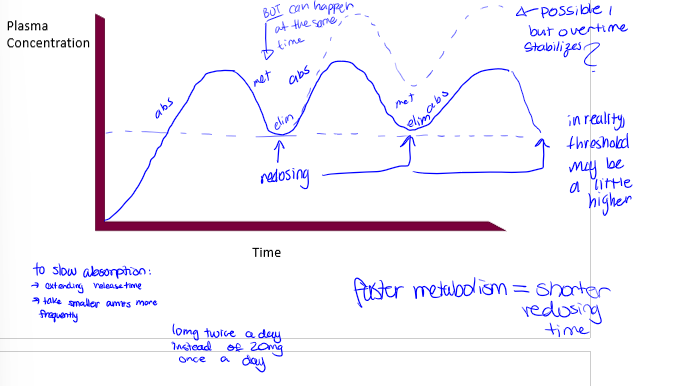
redosing for transdermal drugs with examples
time is relative to the dosing scheme, and time will be long for patches. examples are: nicotine= 24hrs; pain patches=3-7 days; hormones=3-4 days; scopolamine=3 days; nitroglycerine=12-14 hrs, then off for 10-12 hrs
other GI based RoA
sublingual and suppository
sublingual RoA
type of GI RoA. under the tongue, rapid dissolution and uptake bc mucous membrane very thin with high capillary density, oral drug that wont be destroyed with stomach acid
suppository
type of GI RoA. rectal application, no passage through stomach (avoiding stomach acid); useful with vomiting, unconscious patients, and infants who cannot swallow; absorption can be erratic, compliance is highly cultural (EU vs NA)
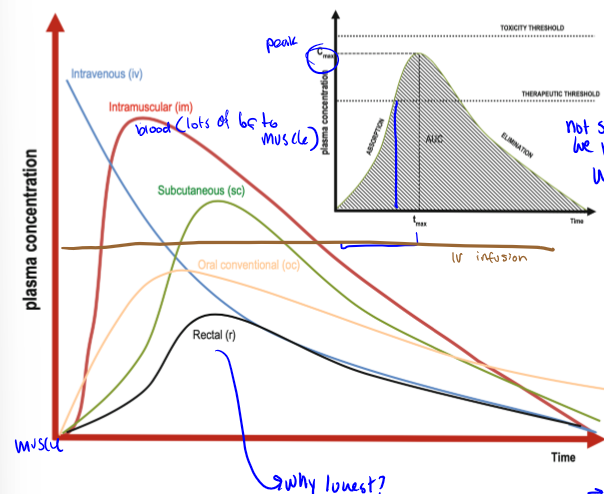
ideal pharmacokinetic curves of IV bolus, IM, SC, OC, IV infusion, and R
IV infusion PK curve
constant plasma concentration, injected amount is initial plasma amount
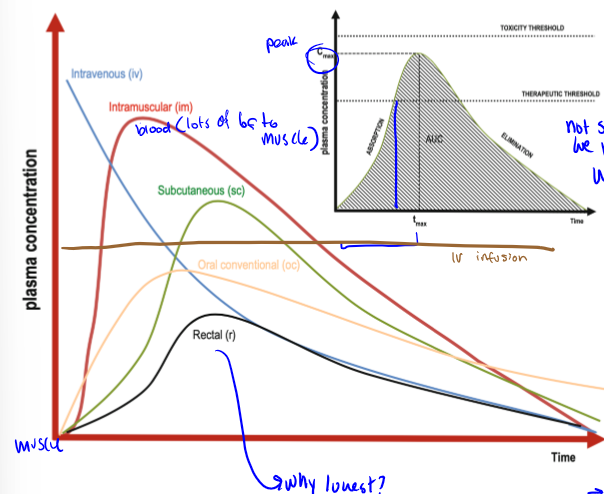
why are PK curve peaks different for different RoAs
becasue of different barriers, depends on the action that you are trying to get
parent compound
the compound that is actually given to the patient, may not be the compound which is absorbed (metabolites are often the compounds absorbed)
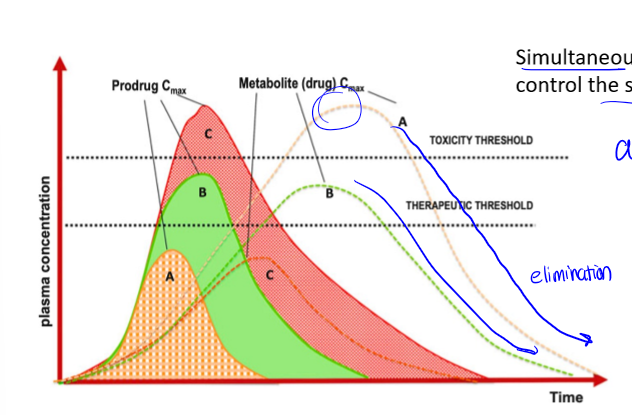
PK curve with metabolites
if drug is metabolized, there is an additional curve for the metabolite. if drug is not metabolized, whole right side of peak is excretion
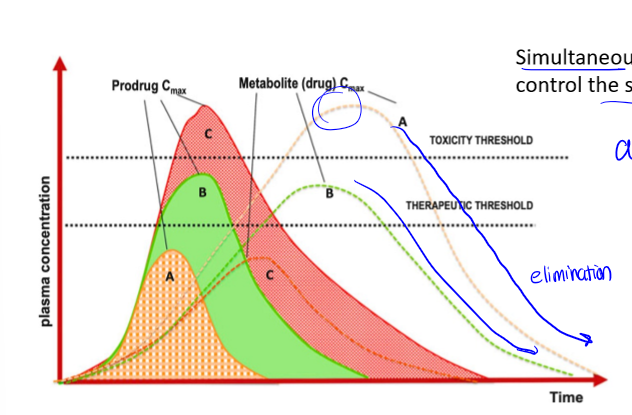
half life of drugs
drugs with a faster peak (IV) have a shorter one. drugs with a slower peak (po/pr) have a later one
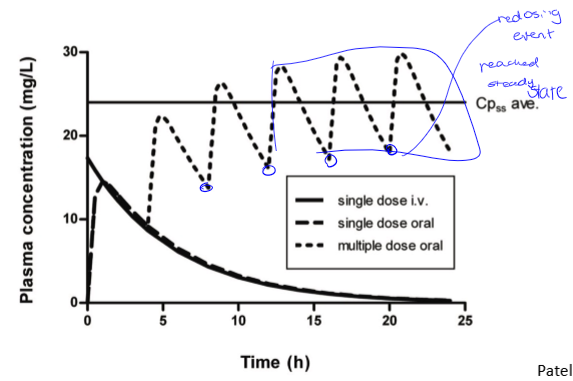
single vs multiple dose oral
redosing events are at valleys. curve is the same dose for each remedication event. may require changes in initial dose to get a steady state quickly. there is a loading dose and a maintenance dose
loading dose
large dose used to kickstart an oral medication when state of health is bad so patient needs therapeutic effect to occur right away and not after multiple doses. should only be used at the beginning to begin. eg, 1g when mdose is 500mg and dose interval is every 6 hours
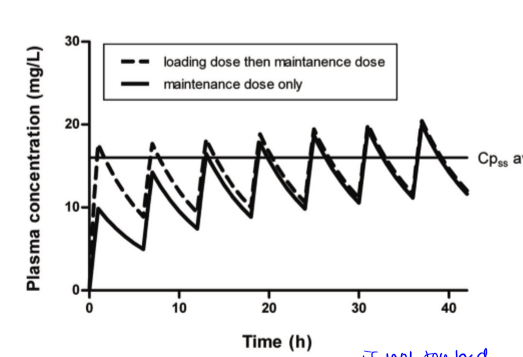
maintenance dose
given after loading dose or sometimes patient starts with mdose if their case is not critical
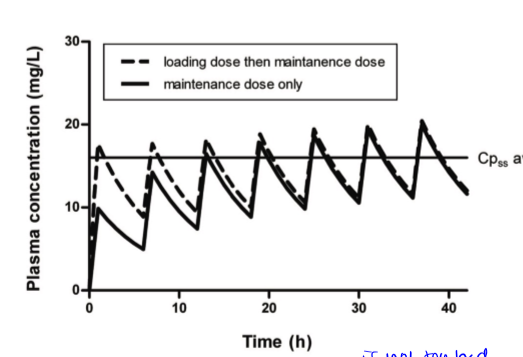
multiple dosing, continuous vs intermittent
want drug conc to be steady, so continuous is best so there is no going below therapeutic effect
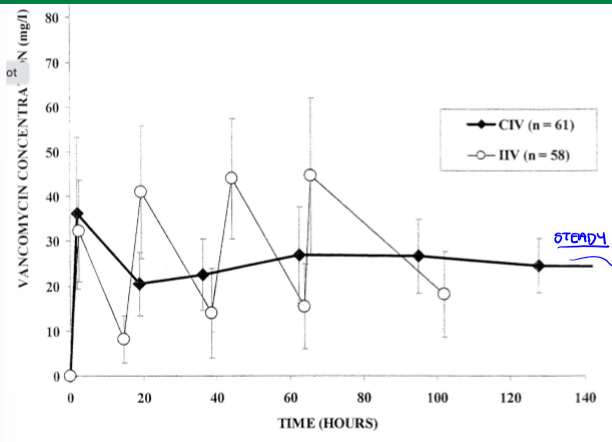
inhalation RoA
compliance slightly lower than oral medication. best application is for asthma. propellant might be required; mists, dry powders; SA and particle size important for absorption; lungs: absorptive barrier is epithelial lining of lower airways, avoid mouth and upper airway; nasal: absorptive barrier is epithelial lining of nose and sinuses, great potential for vaccine delivery
benefits of nasal vaccines
great potential bc right at source (respiratory cells), shelf stable bc vaccine needs to be refrigerated, better for parts of world with less cooling space, training is easier, children would rather inhale
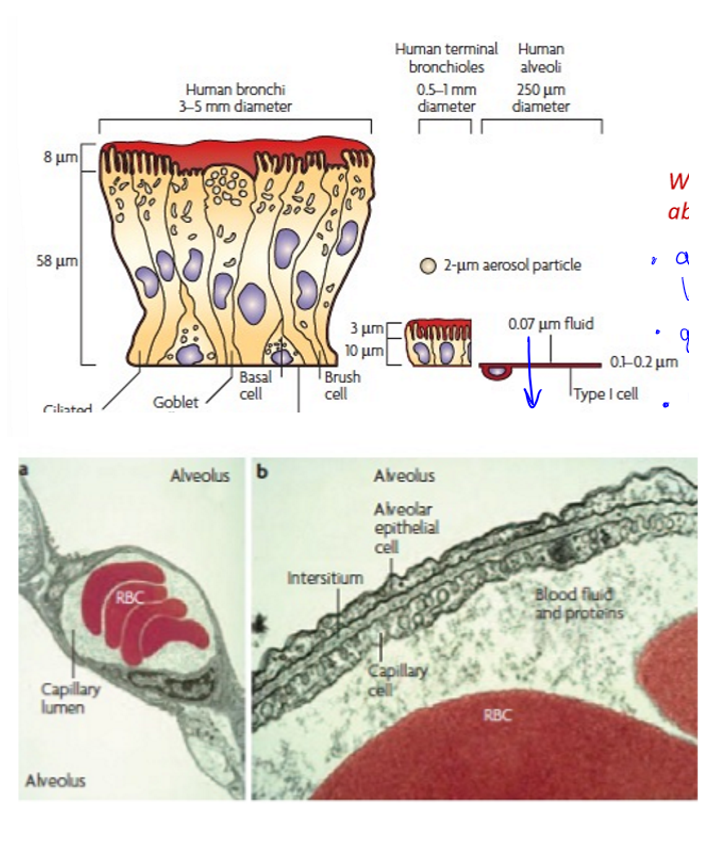
respiratory epithelial cells
human bronchi thickest, next is terminal bronchi much thinner, and finally alveolar which is thinnest so would have the best absorption, would go straight to bloodstream, large surface area
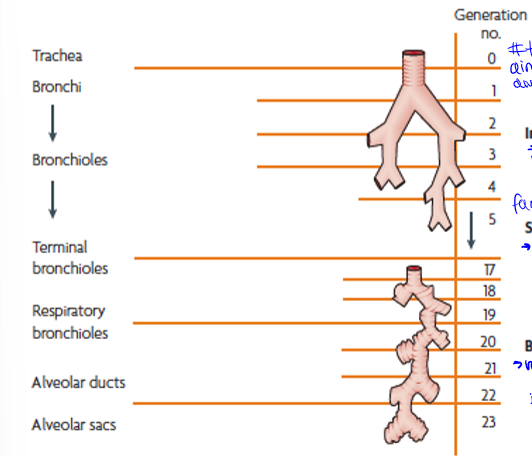
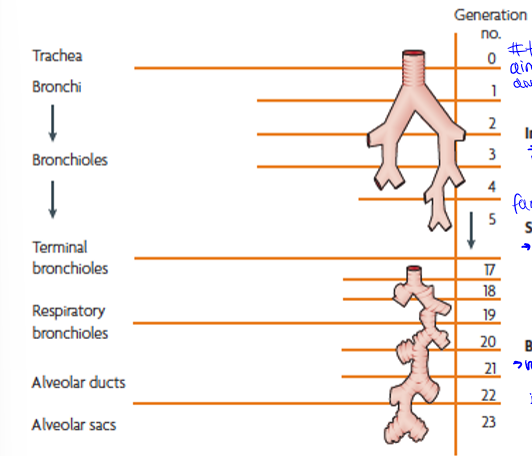
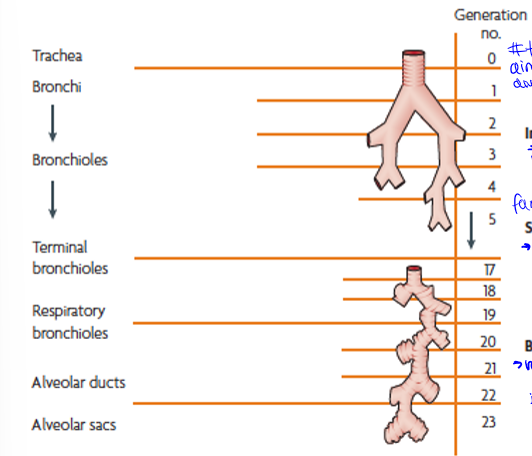
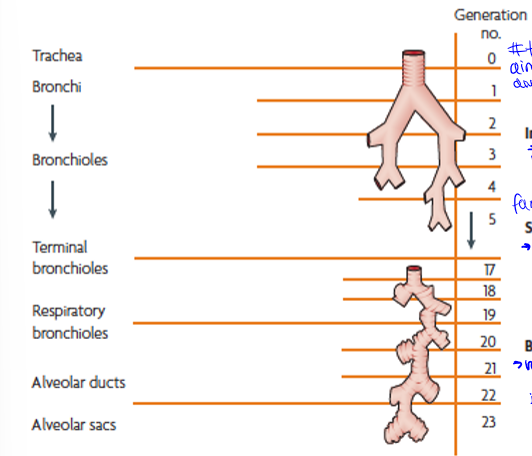
particle motion down the respiratory system
trachea to terminal bronchi, terminal bronchi to respiratory bronchioles, and respiratory bronchioles to alveolar sacs. generations are the amount of times they branch
type of particle motion in upper airways
generation 0 to about 5, impaction (inertia): large particles into walls, slow down, then stuck
type of particle motion in bronchioles
generations 5 to 20, sedimentation (gravity): next largest sizes, g pulls out of air into walls of vessel
type of particle motion in alveoli
generations 20 to the end (23), brownian diffusion (random motion of particles): small particles bring into contact with alveoli
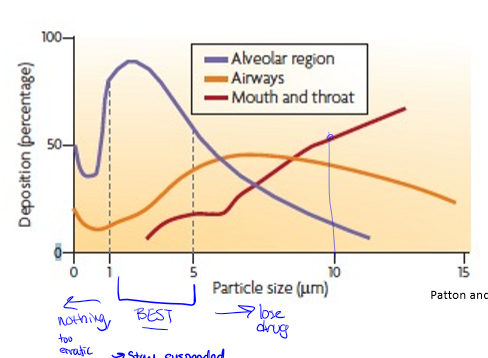
particle deposition by size in inhalation drugs
best sized particle would be best absorbed in alveoli at 1-5 μm
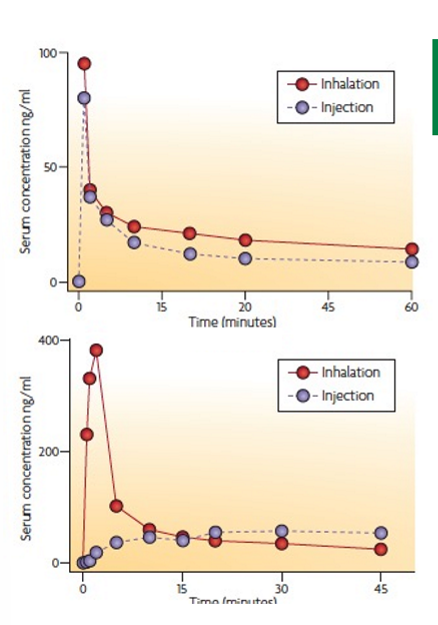
morphine vs rizatriptan drug inhalation case study
morphine human subjects, rizatriptan dog subjects. inhalation sometimes better in some drugs over others due to particle size. morphine inhalation dose twice as high as injected dose, rizatriptan injected dose twice as high as inhalation dose
nebulizer
inhalation RoA with help. drug is contained in mist droplets, inhaled into drugs, used for volatile drugs. small mist droplets travel farthest
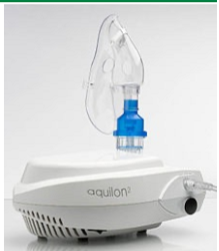
advantages of using a nebulizer
allowing drug to reach deep into lungs, useful for treatment of children of of those with reduced lung function (not active/forceful breathing) as they can sit with mask
disadvantages of using a nebulizer
not the best for active children, harder for some people to use, uses a battery, a lot to carry around so better used at home or at a clinic rather than portable
inhalation metered dose inhaler
provides dosed medication to lung, contains liquified gas propellant and drug, propellant in droplets which evaporates to provide aerosol of micrometer drug particles. particle size determines site of absorption. inhaler is angled so that mouth deposition is reduced

advantage of metered dose inhaler
convenient, simple, portable, provides straightforward way to get drugs into lungs in short amt of time, fast dosing (within mins), pt compliance tends to be high
disadvantage of metered dose inhalor
coordinated and forceful breathing (must inhale at appropriate time that you depress inhaler
aerochamber
that big wonky thing that I used. helps get around coordinated breathing
drug formulations
related to RoA, routes may have more than one formulation, and it may alter drug solubility, bioavailability (how much is absorbed), and drug stability. choice of formulation depends on: age of pt, stability of drug, delivery issues (eg difficulty swallowing), flexibility needs in dosing, and risk of non-compliance. different chemical substances are combined to final medicine product: active drug and non-active components (can change inside with help of enzymes)
metformin and release
diabetes drug, usually taken twice a day, but many people reported GI tract issues so they would stop taking it. instead, Drs gave them extended release drugs that they’d take only once a day