Lecture 6: Parasitology II
1/66
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
67 Terms
Vector borne
diseases transmitted by living organisms, called vectors, like mosquitoes, ticks, and flies, which spread pathogens from one host to another
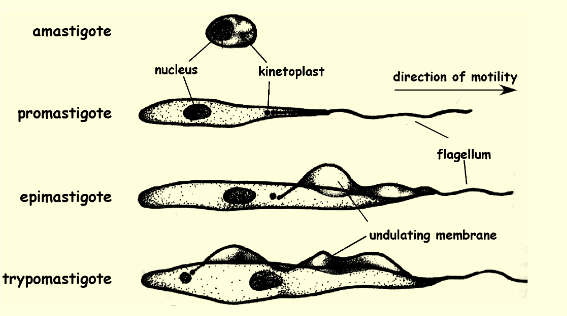
Epimastigote location and function
a motile stage in the life cycle of some protozoan parasites by a flagellum that extends from the anterior of the cell and is attached to the body for part of its length by an undulating membrane found posteriorly host insect midgut and is dividing form
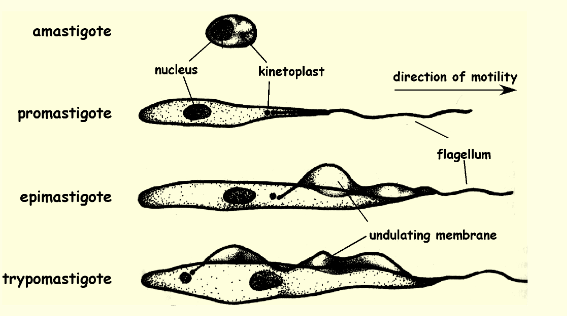
Metacyclic trypomastigote location and function
found in insects gut/feces and is the infective stage of the parasite, the form that’s released from the insect vector
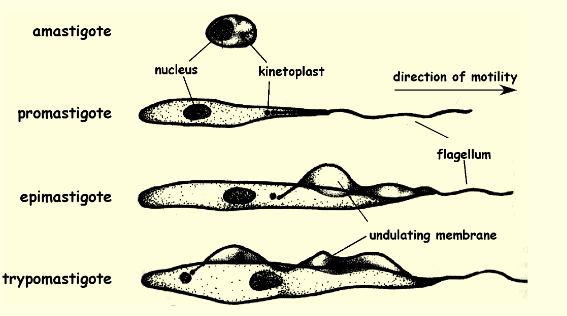
Amastigote location and function
a non-motile, ovoid parasitic cell that is the intracellular stage in the life cycle of certain protozoa and found in human cells and intracellularly divide
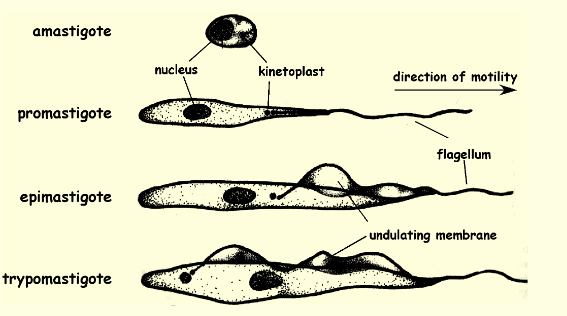
Trypomastigote location and function
a motile, flagella posteriorly w/ undulating membrane, parasitic protozoan that is the form of the parasite found in the blood of an infected host and found in human bloodstream and circulate and infects bugs
Kinetoplastid protozoa
group of flagellated microorganisms that are distinguished by a unique organelle called a kinetoplast
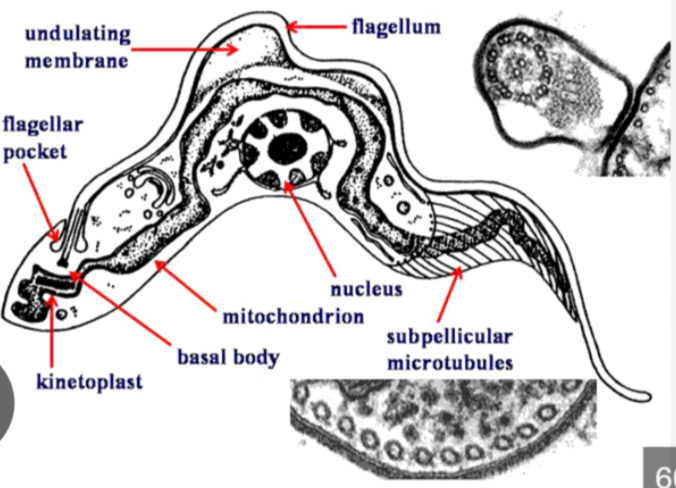
kinetoplast
mass of mitochondrial DNA located at the base of their flagellum
Kinetoplastid Protozoa species
Leishmania, Trypanosoma cruzi, and Trypanosoma brucei
Leishmaniasis
organism leishmania and is found in blood and tissues
Leishmaniasis 3 main forms
Cutaneous, mucocutaneous, and visceral
Leishmaniasis Cutaneous
causes skin sores that can develop into ulcers and abundant amastigotes in lesions
Leishmaniasis Cutaneous Treatment
spontaneous healing; strain-specific immunity; scarring
Leishmaniasis mucocutaneous
starts out like cutaneous more severe form that can cause destructive lesions in the nose, mouth, and throat, amastigotes in lesions
Leishmaniasis mucocutaneous Treatment
chemotherapeutic cure leads to immunity or antimonials but not always effective
Leishmaniasis visceral
the most serious, affecting internal organs and potentially causing death if untreated, with symptoms like fever, weight loss, and an enlarged spleen and liver
Leishmaniasis visceral Treatment
chemotherapeutic cure leads to immunity or miltefosine (drug)
Leishmaniasis reservoir and transmission
Domestic and wild animals like dogs and rodents, spread by vector borne sandfly
Leishmaniasis Epidemiology
tropical and subtropical regions and middle east b/c contact with sandfly and reservoir habitat
Leishmania: Promastigote
The flagellated (single anterior flagella), motile stage of Leishmania found in the sandfly vector, this is the infective form that lives extracellularly in sandfly’s gut
Leishmania: Amastigote
The non-flagellated, intracellular stage of Leishmania found in hosts, it resides and multiplies inside macrophages
Proboscis
elongated, tubular mouthpart of the sandfly (or other insects) used for piercing the skin and sucking fluids, this is used to inject promastigotes in human skin
Leishmania Life Cycle
1.Sandfly bites → injects promastigotes (motile) into human.
2.Promastigotes → enter macrophages → become amastigotes (non motile).
3.Amastigotes multiply → infect new cells → cause disease.
4.Sandfly bites infected human → ingests amastigotes
Leishmania Life Cycle summary
In sandfly: amastigotes → promastigotes → migrate to proboscis
Cycle repeats
Leishmania diagnosis
amastigotes in needle biopsies or aspirates
African Trypanosomiasis location and transmission
Blood, lymphatic, and tissues and spread by vector bornes Tsetse fly
African Trypanosomiasis epidemiology
exclusively sub-Saharan; endemic in tsetse habitat
African Trypanosomiasis prevention
vector avoidance, vector control and monitor susceptible population, aggressive treatment
Trypanosoma brucei is a
the protozoan parasite that causes African trypanosomiasis (African sleeping sickness)
Trypanosoma brucei alternates b/t 2 hosts:
tsetse fly which is the vector and a mammalian host (including humans and animals)
Trypomastigotes
one of the main forms of Trypanosoma species and are flagellated (motile), elongated stage found in the bloodstream of hosts
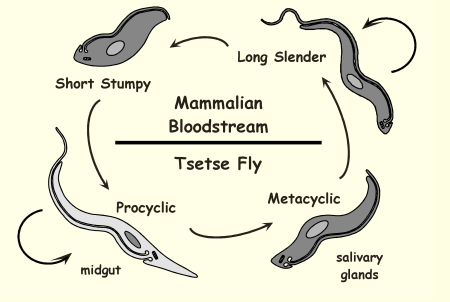
Life Cycle of Trypanosoma brucei
Tsetse fly bites and injects metacyclic trypomastigotes → bloodstream infection → slender forms multiply → stumpy forms ingested by new fly and transform in midgut → mature in salivary glands and infect next mammal.
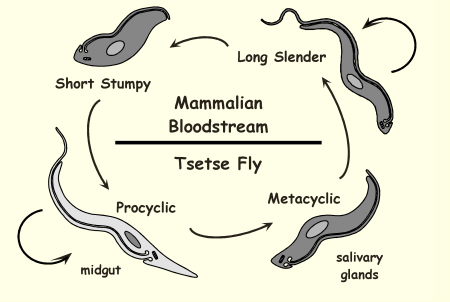
Life Cycle of Trypanosoma brucei: Long slender
host is mammals, dividing trypomastigote and causes infection and evades immune system
This shape allows the parasite to express VSG - antigen variation
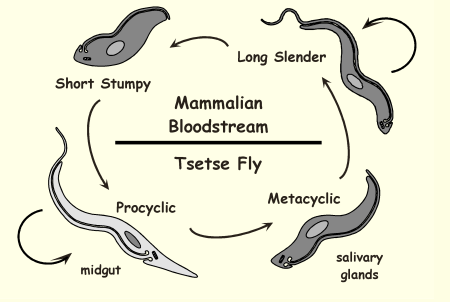
Life Cycle of Trypanosoma brucei: Short stumpy
host is mammal, non dividing trypomastigote, and is infective to tsetse fly
too small to divide, specifically infect tsetse fly not humans, job is to transmission - stress resistant
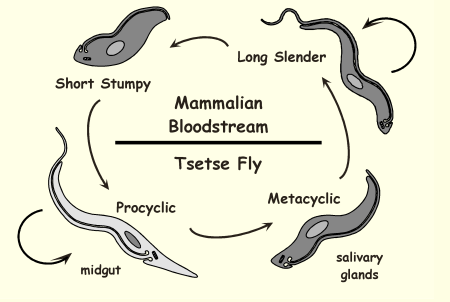
Life Cycle of Trypanosoma brucei: Procyclic
Host is fly midgut (only!!), job is dividing trypomastigote, and is adapted to fly environment
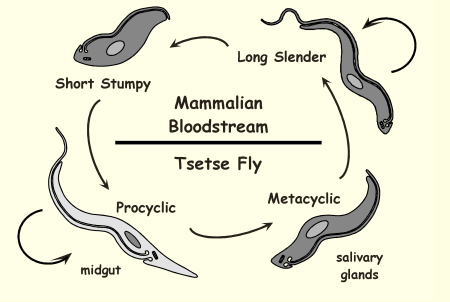
Life Cycle of Trypanosoma brucei: Metacyclic
Host is fly salivary glands, Infective trypomastigote, and is then transmitted to mammal
-non dividing, job is to infect humans
Why does African Trypanosomiasis need 2 hosts ?
the parasite needs a mammal (human or animals) to multiply and a tsetse fly to complete its life cycle
African Trypanosomiasis Immunity
initial humoral response to immunosuppression but then immune evasion by antigenic variation
African Trypanosomiasis Diagnosis
direct examination of blood, lymph, and CSF and card agglutination test, T. gambiense only
African Trypanosomiasis Treatment
if infection is early simple drugs are good, if infection is late the drug needs to cross blood brain barrier
Antigenic variation in Trypanosoma brucei (African)
covers its surface with a dense coat of variant Surface Glycoprotein (VSG), its able to constantly switches which VSG gene it expresses antigen evading the immune system
-happens only in slender form
Winterbottom’s Sign
swelling of lymph nodes in the back of the neck, a key early symptom of African trypanosomiasis (sleeping sickness) caused by the parasite Trypanosoma brucei
South American Trypanosomiasis (AKA chaga’s disease)
Caused by Trypanosoma cruzi and reservoir are rats, cats, dogs, and opossum
South American Trypanosomiasis location and transmission
Found in blood, lymphatics & tissues and spread by vector borne reduvid bugs
South American Trypanosomiasis Prevention
vector control; good housing; screen blood supply

Life Cycle of Trypanosoma cruzi
alternates between insects and humans (2 hosts), using amastigote and trypomastigote forms to multiply and spread. Infection occurs when infective feces of the reduvid bug contaminate a bite wound, leading to Chagas disease
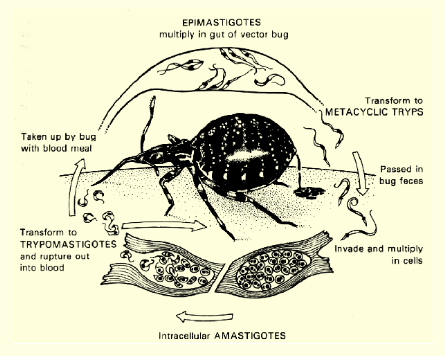
Life Cycle of Trypanosoma cruzi specific
Metacyclic trypomastigote: delivered in bug feces that invades human cells
Amastigote: inside host cells, no flagella, and major dividing stage in humans
Bloodstream trypomastigote: non-dividing form in blood and ingested by reduvid bug
Epimastigote: in insect midgut where it divides in bug and comes back into metacyclic trypomastigote
South American Trypanosomiasis Acute pathology
2-4 months fever, chagoma (Romana’s sign), hematogenous spread; circulating trypomastigotes
- severe in children;
South American Trypanosomiasis Chronic pathology
10-20 years no trypomastigotes but persistent amastigotes, tissue damage, and heart attack is common
South American Trypanosomiasis Diagnosis
acute: trypomastigotes in blood
chronic: serology, xenodiagnosis
schizogony
asexual reproduction by multiple fission, found in some protozoa, especially parasitic sporozoans
sporulation
in sexually reproducing parasites, sporulation can result from the fusion of gametes (formed from haploid gametophytes) to create a diploid zygote,
Apicomplexan Parasites Characteristics
Obligate intracellular parasites, apical intracellular organelle, and lifecycle is asexual and sexual (found in intestinal epithelial of host)
Toxoplasmosis
caused by organism Toxoplasma gondii and found in tissue/blood
Toxoplasmosis reservoir and prevention
cats, mice, sheep and prevented by avoidance behavior by at risk populations
Toxoplasmosis transmissions
spread ingestively: fecal/oral or undercooked meat
spread transplacental: congenital (birth) infection
Toxoplasmosis Epidemiology
world wide disease in developing countries for undercooked meat, hygiene, congenital, and cats
oocysts
environmental, infective stage of Toxoplasma gondii, it is a thick highly resistant structure that allows parasite to survive and spread infection
Cats
are the only definite host were toxoplasma can undergo sexual reproduction, only ones that produce oocysts
Toxoplasma gondii reproduces
sexually in cats (in intestine only) and asexually in other animals and humans, infection is spread via cat feces (oocytes) or undercooked meat
Toxoplasma gondii has 2 life cycles
Natural cycle: cats definite host
Incidental cycle: human incidental host
Rodents/birds: intermediate host
Toxoplasma life cycle: cats
Toxoplasma undergoes sexual reproduction in cats where the parasite multiples in the gut, cats shed immature oocysts in feces, then intermediate hosts rodents/birds ingest oocysts and parasites convert into tachyzoites which then form tissue cysts (bradyzoites)
Toxoplasma life cycle: humans
accidental hosts eat undercooked meat containing tissue cysts, parasites turn into tachyzoites that spread thru body, and the cycle ends here b/c these hosts cant shed oocysts
Two Phases of Toxoplasma Infection
Tachyzoite and Bradyzoite
Toxoplasma Tachyzoite
rapid replicative form during initial acute infection caused by reactivation of dormant cysts, and control by primary immune system
- can cross placenta, causes encephalitis in AIDS
Toxoplasma Bradyzoite
encysted slow growing form during dormant phase, source of reactivation throughout life-long infection, and control by memory immune response
Toxoplasmosis Diagnosis
serology; indirect immunofluorescence (IFA) and seizure in AIDS: MRI, treat, repeat MRI
Toxoplasmosis Treatment
essential for immunocompromised or active chorioretinitis and prevented by breaking the transmission cycle