2010 CBF - Membrane Proteins
1/28
Earn XP
Description and Tags
It's the membrane protein lecture!
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
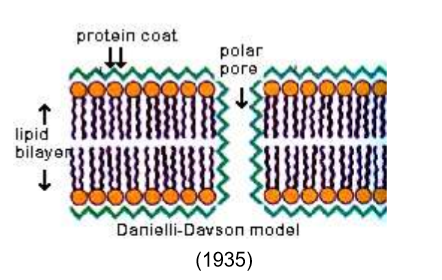
What’s the difference between Gorter and Grendel’s membrane model vs the Danielli-Davson model?
The Danielli-Davson model was essentially the same, but they introduced a protein coat lining the lipid membrane and polar pores. People felt comfortable with it because it appeared to match microscopy and added rigidity to the membrane.
What two experiments smacked the gob out of Danielli-Davson?
Frye and Edidin
Pinto da Silva and Branton
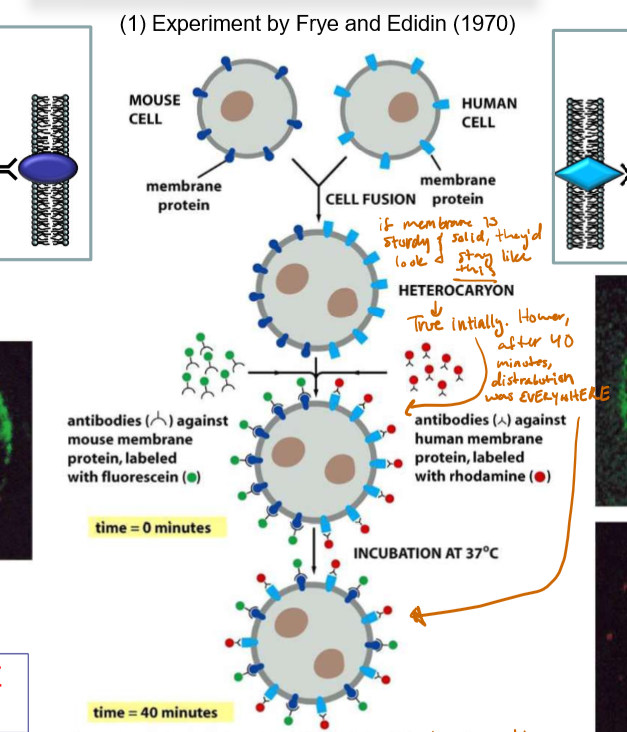
Describe the Frye & Edidin experiment and its significance.
A mouse cell with membrane proteins dyed green (and excited with blue light to fluoresce) and a human cell with membrane proteins dyed red (and excited with green light to fluoresce) were fused. If the membrane was stationary, the result would be a perfectly half-green-half-red cell.
Initially, it appeared to be this way, but after 40 minutes the cell proteins became dispersed throughout the membrane, supporting the idea the membrane was NOT stationary, but rather SLOSHY ASF.
Modern Frye-Edidin Experiment and its superiority?
The membrane photo-bleaching experiment: FRAP - Fluorescence Recovery after Photobleaching
definitively quantitative (measure the percent of recovery)
No need for rat/people blobs
Easier to differentiate stationary proteins
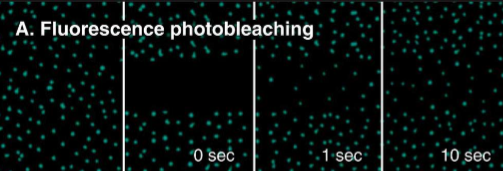
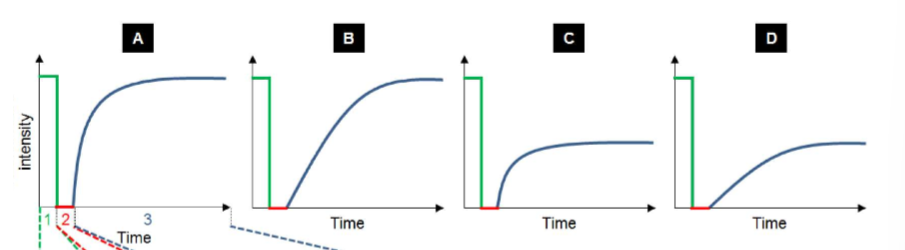
Describe these FRAP graphs
Green peak: Pre-bleaching protein max
Red line: Fluorescence in that area
A) Fast with maximum recovery
B) Slow with maximum recovery
C) Fast with limited recovery
D) Slow with limited recovery
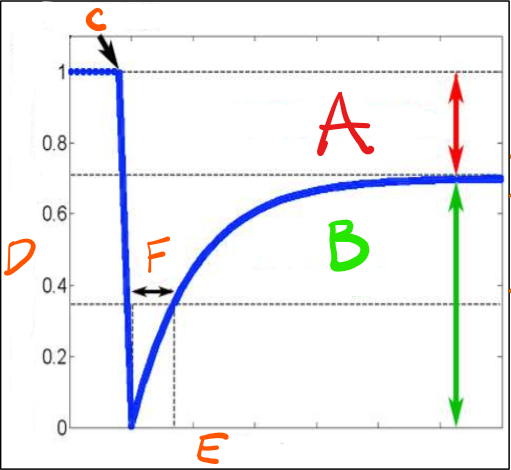
Explain the labels
A) Immobile Fraction - amount of protein left stationary after being bleached. These could be proteins attached to the cytoskeleton.
B) Mobile Fraction -The total mobile proteins in the plasma membrane (likely peripheral proteins)
C) Point of bleaching - The initial laser bleaching point.
D) Fluorescence in the bleached area - The initial level of fluorescence in the bleached area.
E) Time - The entire time it takes to recover a set level of fluorescence in that bleached area.
F) Time it takes for half of the membrane protein to recover fluorescence - Calculate the slope from this point. Slow recovery would have a large slope, fast recovery would have a small slope.
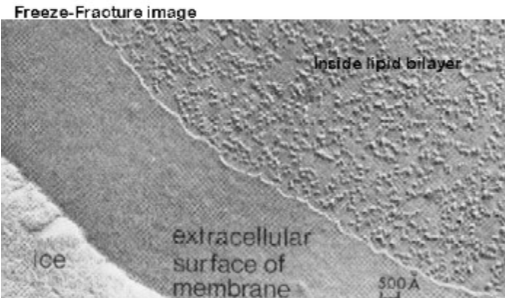
Explain the Pinto de Silva and Branton experiment and its significance.
A frozen bundle of cells is chipped to split the leaflets of the plasma membrane. Then further ice was evaporated to expose the edges of the cell membrane that weren’t split. Then heavy metals were used to etch the surfaces of the cells.
It showed bumps along the surfaces of the cells, which were proteins that thus implied they crossed the bilayer (there were bumps in one leaflet and pits in the other. Also, this only showed transmembrane proteins, not peripheral).
This thus proved that proteins did not coat the bilayer but rather, crossed it.
Who is the polysaccharide coat Glycocalix?
Polysaccharides found using special sugar dyes on the outside of the plasma membrane.
They coat the transmembrane proteins.
Roles of glycocalyx?
Mechanical stability - cushion against mechanical impacts
Chemical protection - Takes the brunt of chemical reactions that take place in the extracellular matrix, protecting membrane proteins
Identification - One form of “caller ID” in cell-to-cell communication
Cell-cell repulsion or, sometimes, adhesion - The sugars, negatively charged, prevent unwanted cell interactions
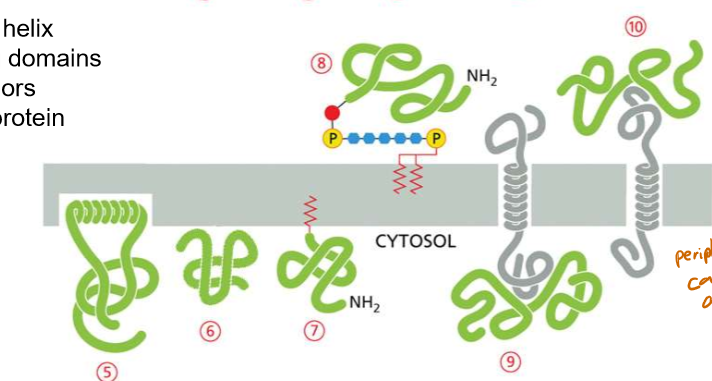
How many amino acids are in an alpha helix for any given transmembrane protein?
20
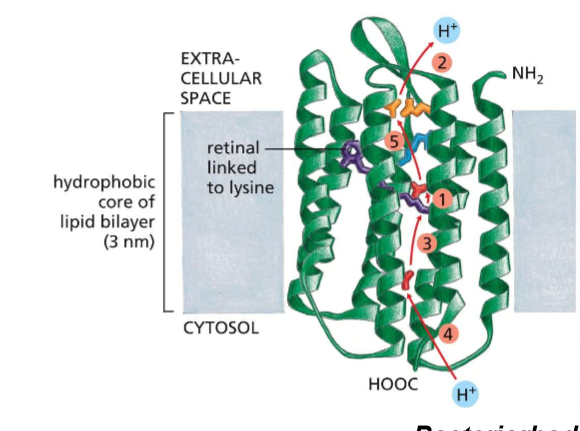
What differs about the interactions between multi-pass and single-pass membrane proteins?
Multi→ interacts with the cell membrane AND each other; therefore, they may have amphipathic, hydrophillic, sulfuric, and hydrogen-bonding based interactions WHILE being in the membrane— amino acids will not be exclusively non-polar.
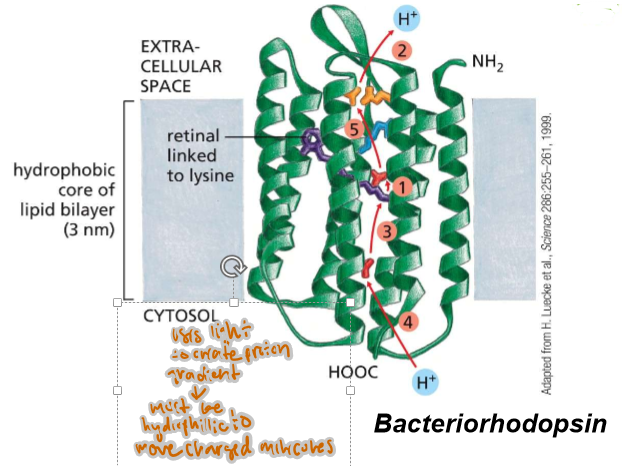
How may passes are in protein A? In protein B?
A) 1
B) 7

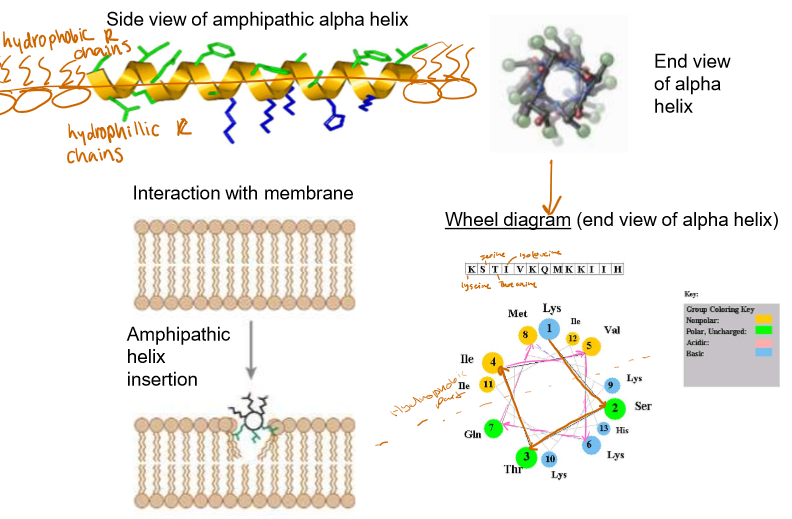
Discuss the structure of an amphipathic alpha helix in a peripheral protein
Internal layer side chains are hydrophobic
Externals are hydrophilic
Looking at the amino acids in a wheel diagram can help you determine how the molecule is embedded.
Discuss the structure of lipid binding peripheral proteins.
Their positively charged ends interact with the negatively charged internal leaflet of the plasma membrane
Often these proteins are recognizing specific lipid heads like PIPs (phoshotidyl inositol phosphates)
Discuss the structure of lipid-anchored peripheral proteins.
They either anchor a protein to the inside of the cell, or the outside of the cell
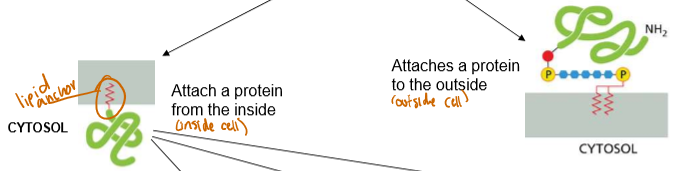
Types of Lipid Anchors
Internal differentiation only, all external lipid anchors attach to proteins willy nilly:
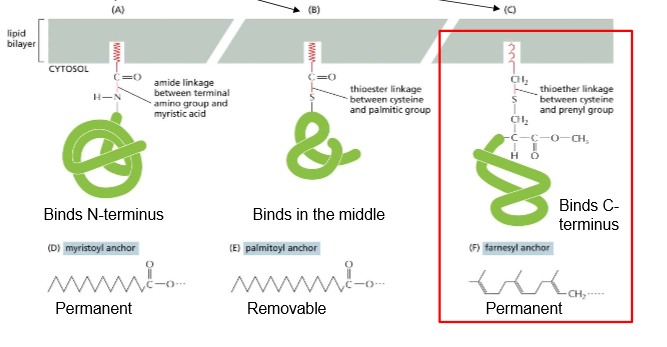
N-Terminus binding:
Permanent
Added when Protein is synthesized
“Middle” Binding:
Only caused by cysteine’s thioester linkage with a palmitic group
Removable
Will diffuse into cytoplasm until needed to attach again
C-Terminus binding:
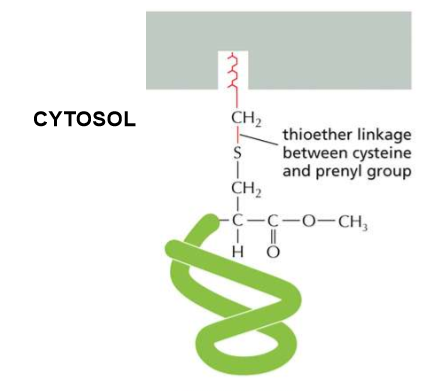
Thioester linkage between cysteine and prenyl (long, hydrophobic) group
C-Terminal Prenylation?
Long, hydrophobic anchors in the cell membrane create a thioether linkage between the cysteine and the prenyl group.
Ex prenyl: farnesyl
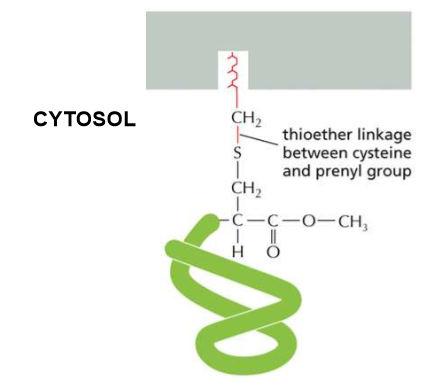
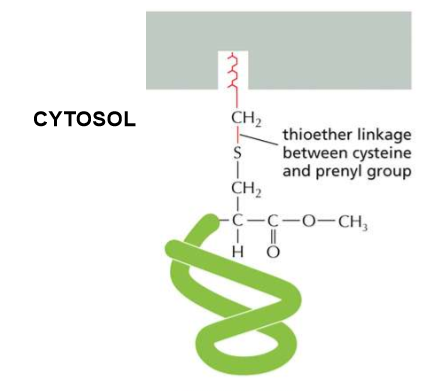
Describe the linkage order of C-Terminal Prenylation
Protein→Cysteine→ two aliphatic amino acids (both hydrophobic and NOT aromatic) and any random amino acid (Caax sequence).
The -aax is cleaved and the amino acid’s carboxyl group is methylated to prevent any further reaction.
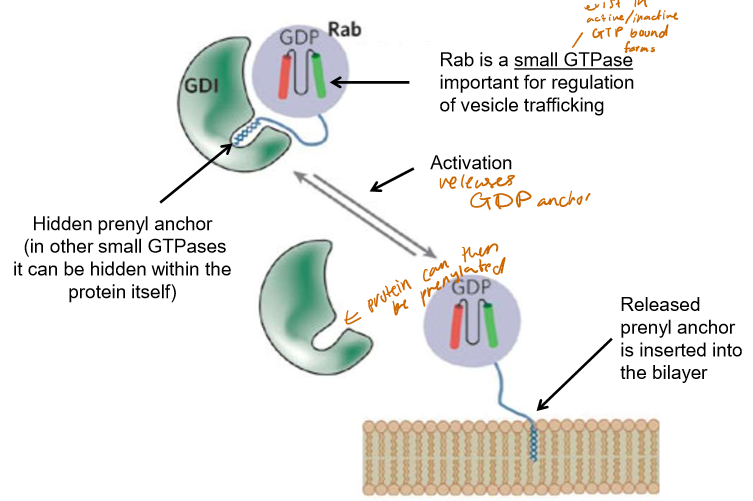
How is Prenylation regulated?
GTPases, such as Rab (important for vesicle trafficking) have prenyl anchors hidden within GDIs or themselves that, when activated, can be inserted into the bilayer.
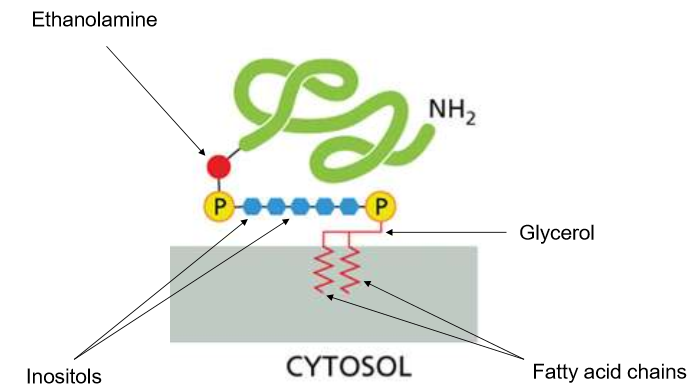
What are GPIs?
Glycosylphosphatidylinositol - a giant “phospholipid” head with two anchors in the bilayer. They interact with the extracellular matrix and often help to identify themselves.
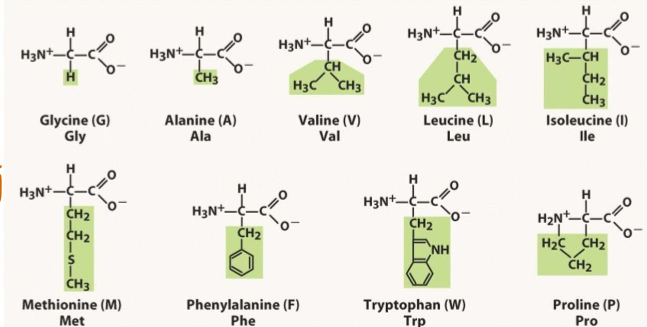
List all the nonpolar amino acids (9)
G
A
V
L
I
M
F
W
P
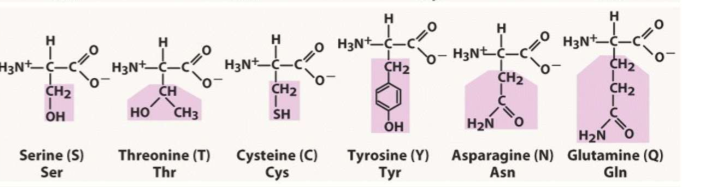
List all the polar amino acids (6)
S
T
C
Y
N
Q
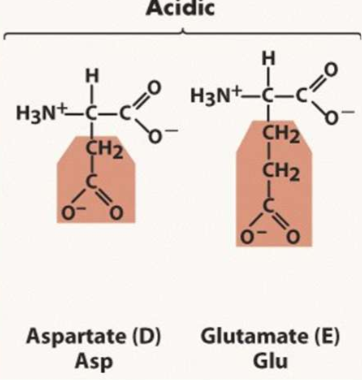
List the negatively charged amino acids
D
E
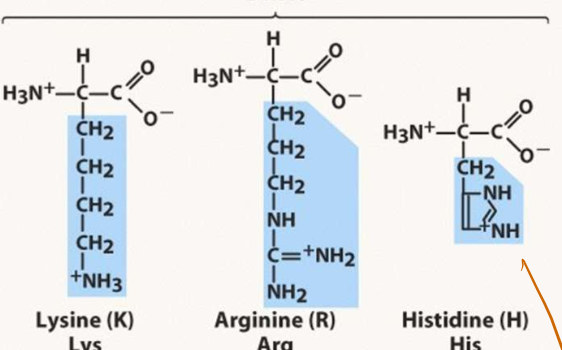
List all of the basic amino acids
K
R
H
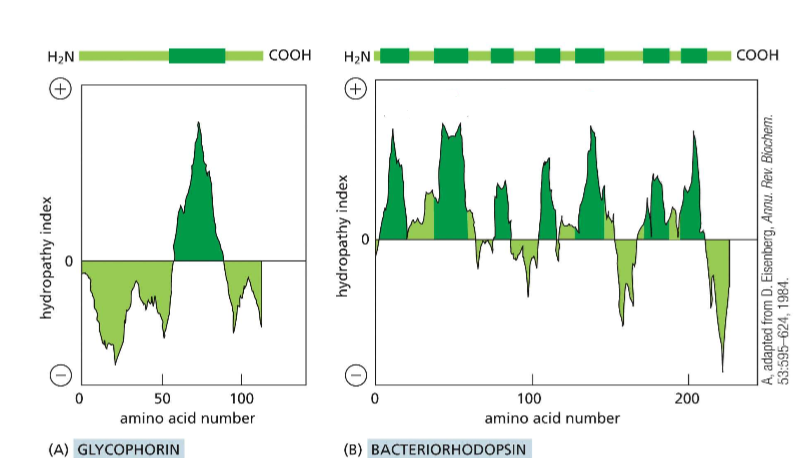
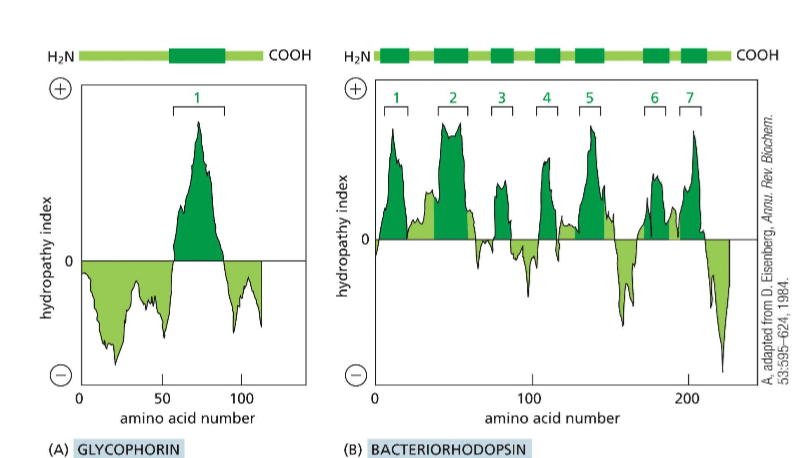
Describe the numerical scale of the hydropathy index
The more positive on the scale, the more hydrophobic the region of the protein is. Negative values indicate hydrophilic protein regions. These can help you determine the positionality of the protein (Transmembrane, membrane-anchored, cytosol-free-floating, etc).
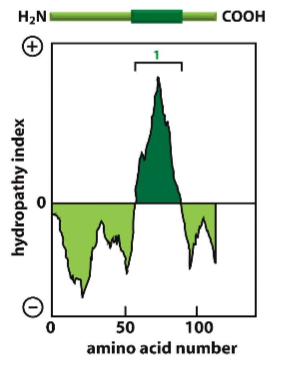
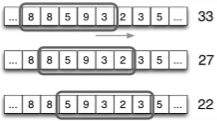
How do you create a hydropathy plot?
It is the addition of a sliding window of individual amino acids to determine regions of hydrophobicity and hydrophilicity.
What happens when GPIs mutate? Use an example from class
If a GPI is unrecognizable, the cell on which it likes can become unrecognizable to the cell environment, and thus can be attacked by immune cells.
In Class Example: RBCs with faulty GPI anchors get SLAUGHTERED by the immune system, causing a myriad of problems.
List the four types of peripheral proteins
Amphipathic helix
Lipid binding domains
Lipid-anchored
Protein-Protein Interaction
List the two types of transmembrane proteins
Single-pass proteins
multi-pass proteins.