General Chemistry for Engineers Midterm 1
1/241
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
242 Terms
chemistry
the study of structure, composition, & properties of matter; energy flow during physical and chemical change
matter
an entity which has mass and occupies space (has volume)
mass
measures amount of matter in grams
analytical balance
utilized to determine mass (in grams) in laboratory setting
weight
measures the earth's gravitational force on an object (measurement of force)
W=mg
g = gravitational acceleration (9.8 m/s^2)
units: pounds (Lbs)
force
an action that causes acceleration or deformation of an object (a push or pull)
Newton's Second Law of Motion
acceleration of an object depends on the mass of the object and the amount of force applied (F=ma);
measured in Newtons (N) which is kg m/s^2
weight is _____________ to mass
proportional
volume
amount of space occupied by matter
typical units: mL, cm^3, or L
displacement
used to determine volume of irregular objects
1.) Fill a graduated cylinder and record volume
2.) Add object to water
3.) Calculate: Vobject=Vfinal-Vinitial
density
amount of matter per unit volume (concentration)
d=m/v
typical units: g/mL, g/cm3, or, for gases, g/L
solids, liquids, and gases ____________ upon heating and ___________________ upon cooling
expand, contract
Temperature affects _____________ in d=m/v. Thus, __________________ ____ _____________ varies with temperature
volume (v), density of matter
Matter is dichotomized into 2 categories being....
purse substances and mixtures
pure substance
material of fixed composition
1.) elements
2.) compounds
elements
pure substances comprised of one type of atom
# of protons
atomic number
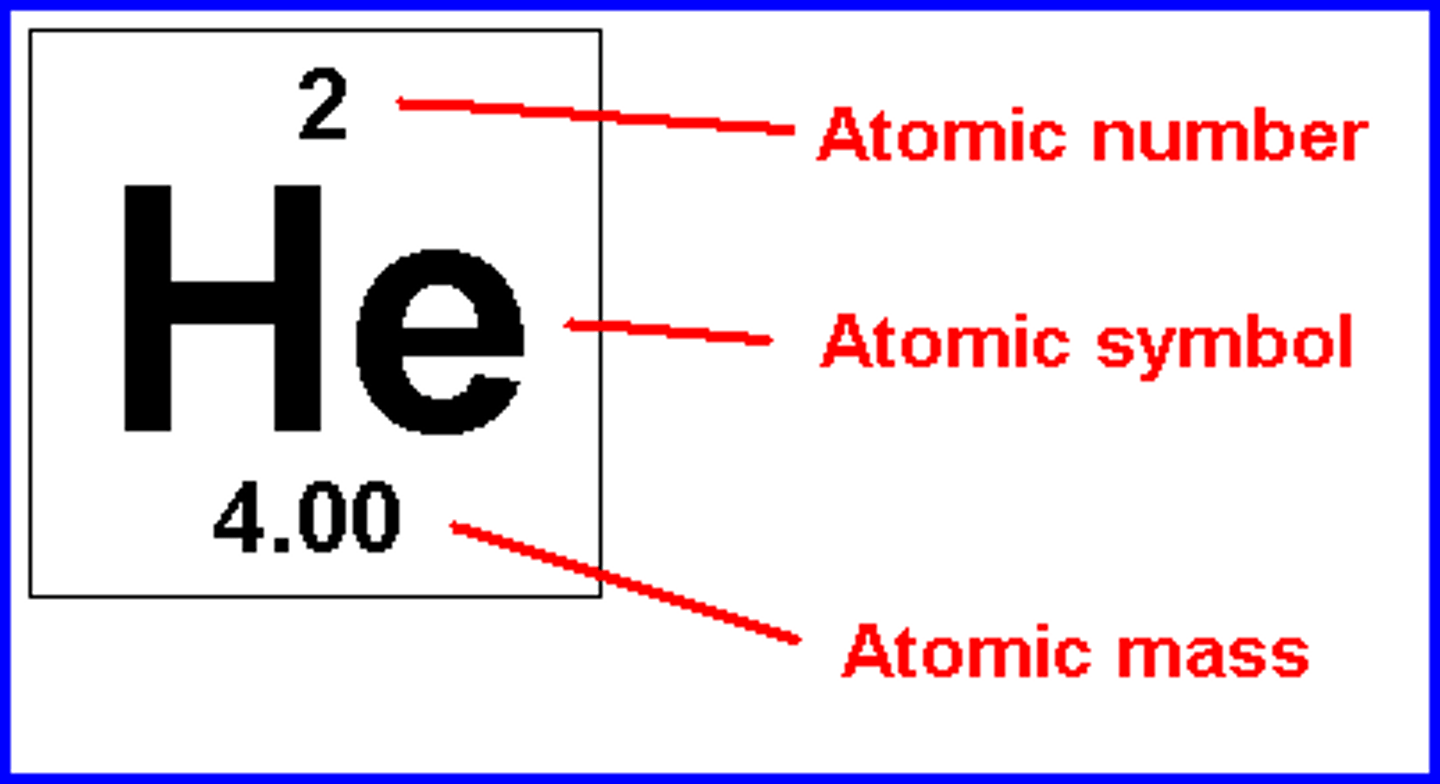
# of neutrons
atomic mass - atomic number
# of electrons
# of protons (+ or - any charges)
atom
smallest particle unit of an element (retains properties of element)
groups
vertical columns
periods
horizontal rows
compounds
pure substances comprised of two or more elements chemically combined in a fixed ratio as a result of chemical reactions between elements
molecule
independent structural unit comprised of two or more atoms that are chemically bound together
molecules can be
diatomic, triatomic, and polyatomic
mixtures
-one or more different substances
-substances that are physically intermingled
-variable composition
-can be homogenous and heterogeneous
homogenous mixtures
-appear as a single, uniform phase
-constituents are not distinguishable
ex: mouthwash, saline rinse
heterogenous mixtures
-appear as a multi-component entity
-boundaries between constitutes visible
ex: sandwich, muddy water
physical change
-phase or state of matter changes
-chemical composition remains constant.
physical change is initiated by...
changes in temperature and pressure
fusion
solid to liquid (melting)
freezing
liquid to solid
vaporization
liquid to gas
condensation
gas to liquid
sublimation
solid to gas
deposition
gas to solid
mixture
-contains two or more components
-components are physically intermingled
-can vary in its composition
physical properties
characteristics of a material when it is not undergoing change
-metals are shiny, malleable, electrically conductive
-ammonia gas has a strong odor
-aluminum oxide is a brittle solid
-chlorine is a pale-green gas
chemical properties
refer to behaviors of matter while undergoing chemical change
-octane is flammable
-iron rusts in the presence of moisture
-copper rooftops turn green over time
-sodium explodes when added to water
4 observations consistent w/ chemical change
1.) temperature change
2.) color change
3.) evolution of a gas
4.) formation of an insoluble solid
temperature
measures
-how hot or cold a substance is
-average particle energy of motion (kinetic energy)
typical units: Celsius and Kelvin
celsius to kelvin conversion formula
Tk = Tc + 273.15
exothermic reaction
-temperature increases
-heat flows from system into surroundings
-system feels warm to touch
endothermic reaction
-temperature decreases
-heat flows from surroundings into system
-system feels cold to touch
color change
indicates system interacts differently with ambient light
evolution of a gas
appearance of bubbles, as a result of combining 2 unalike substances
precipitate formation
in a precipitation reaction, when 2 clear liquids are combined and insoluble solid appears
Law of Conservation of Mass
-matter is conserved in a chemical reaction
-matter is neither created nor destroyed.
Law of Definite Composition
regardless of origin, a compound contains the same elements in the same ratios by mass
% mass of element
% mass of element = (mass element/mass compound) * 100
mass average equation
m ave = (mass 1 amu 1) + (mass 2 amu2)
Law of Multiple Proportions
If two masses of an element react with a defined mass of a second element to form two different compounds... The two masses of the first element form a ratio of small-whole numbers
energy
the ability to do work
work
force applied to an object over a given distance
W=Fd
typical units: m^2/s^2, Newton-meter (N*m), Joule
4 forms of energy
thermal, luminous, electrical, nuclear
Rules for significant figures
- all non zero digits are significant
- zeros between nonzero digits are significant
- zeros preceding nonzero digits are not significant figures
- zeros at the end of a number with a decimal place present anywhere are significant
- zeros at the end of a number with no decimal present are not significant
how many sig figs are in 100?
1 sig fig
how many sig figs are in 0.0410?
3 sig figs
how many sig figs are in 0.0011?
2 sig figs
addition & subtraction w/ significant figures
Watch decimal places!
A sum or a difference is reported to the same number of decimal places as the measurement in the calculation with the fewest decimal places.
Example: Volume = 83.5 mL + 23.28 mL + 106.8001 mL Volume = 213.6 mL
multiplication & division w/ significant figures
Watch total sig figs in each measurement
The product or quotient is reported using the same total number of sig figs as the value entered into the calculation with the fewest
Example: Volume = (9.2 cm)(6.81 cm)(0.3744 cm) Volume = 23 cm3
logarithms w/ significant figures
a calculated log value should contain the same number of decimal places to the decimal as there are significant figures in the value used
Example: log10(3.67x 10-3) = -2.435
accuracy
how close a measurement is to an accepted value
percent error
used to assess how far a measured value is from an accepted value
percent error formula
% error = (measured value - accepted value) / (accepted value) * 100
precision
refers to how close a collection of measured values are with respect to one another
Example: Three different students record the following measurement for a liquid in a graduated cylinder
-32.2 mL
-32. mL
-32.3 mL
Note how close these values are to one another
There is high precision among these measurements
giga-
G 10^9
mega-
M 10^6
kilo-
k 10^3
deci-
d 10^-1
cenit-
c 10^-2
milli-
m 10^-3
micro-
μ 10^-6
nano-
n 10^-9
pico-
p 10^-12
femto-
f 10^-15
International System of Units (SI Units)
MKS provided foundation for this system
Meter (length)
Kilogram (mass)
Second (time)
Ampere (current)
Kelvin (Temperature)
Mole (amount)
Evidence that light behaves as a wave
reflection, dispersion, refraction, diffraction
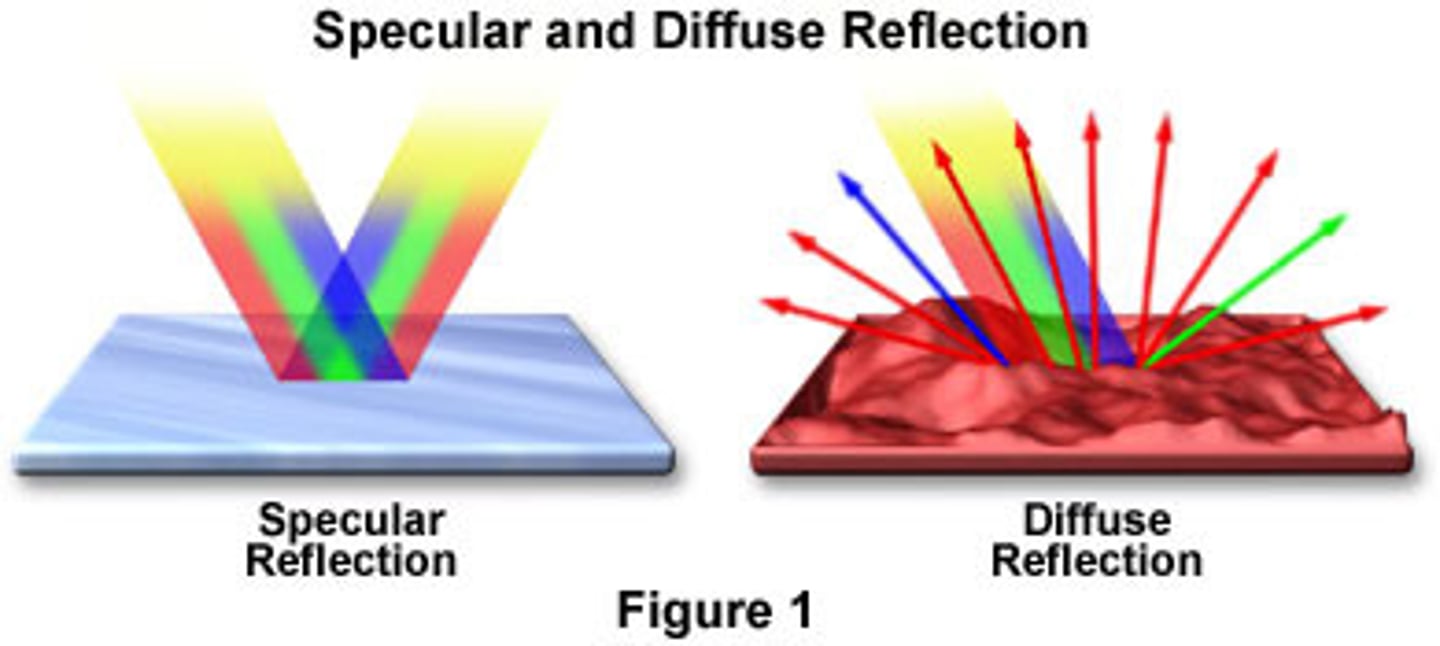
specular reflection
arises from illuminating polished surface (ex: mirror)
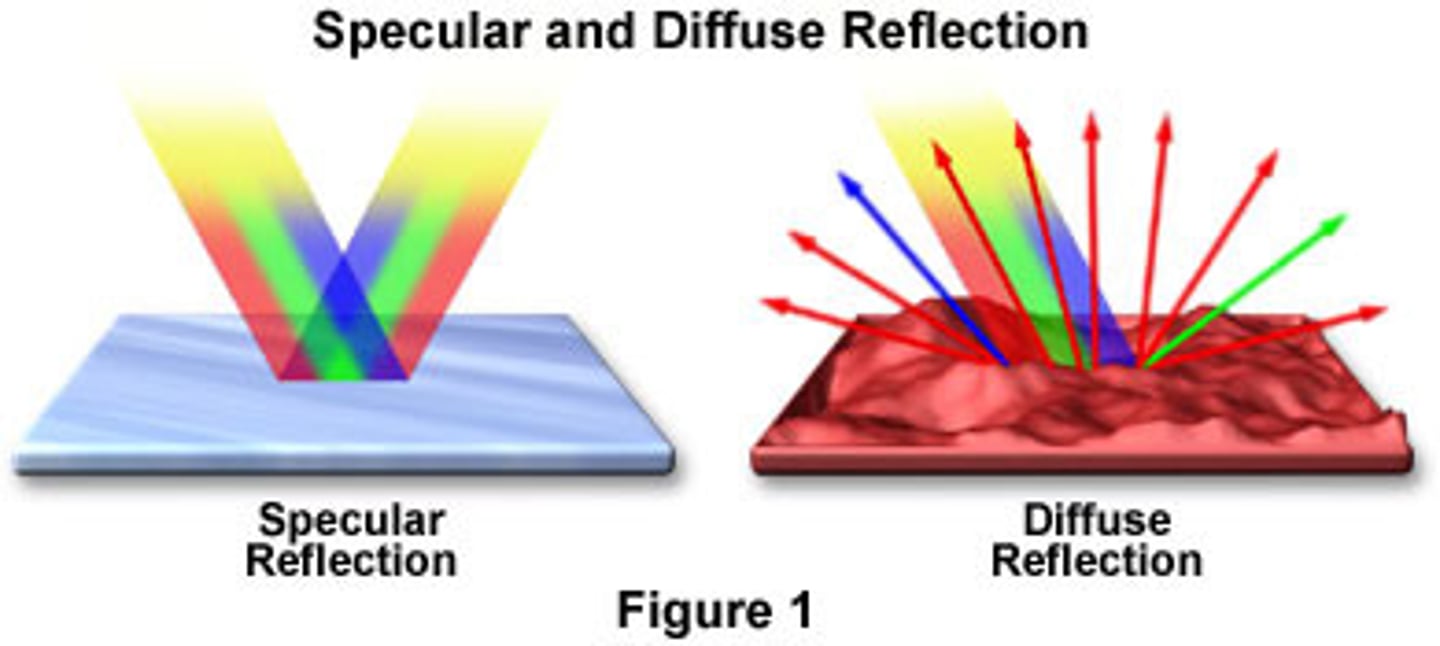
diffuse reflection
arises from illuminating rougher surfaces
dispersion
shining white light through prism separates light into different wavelengths so we see a rainbow
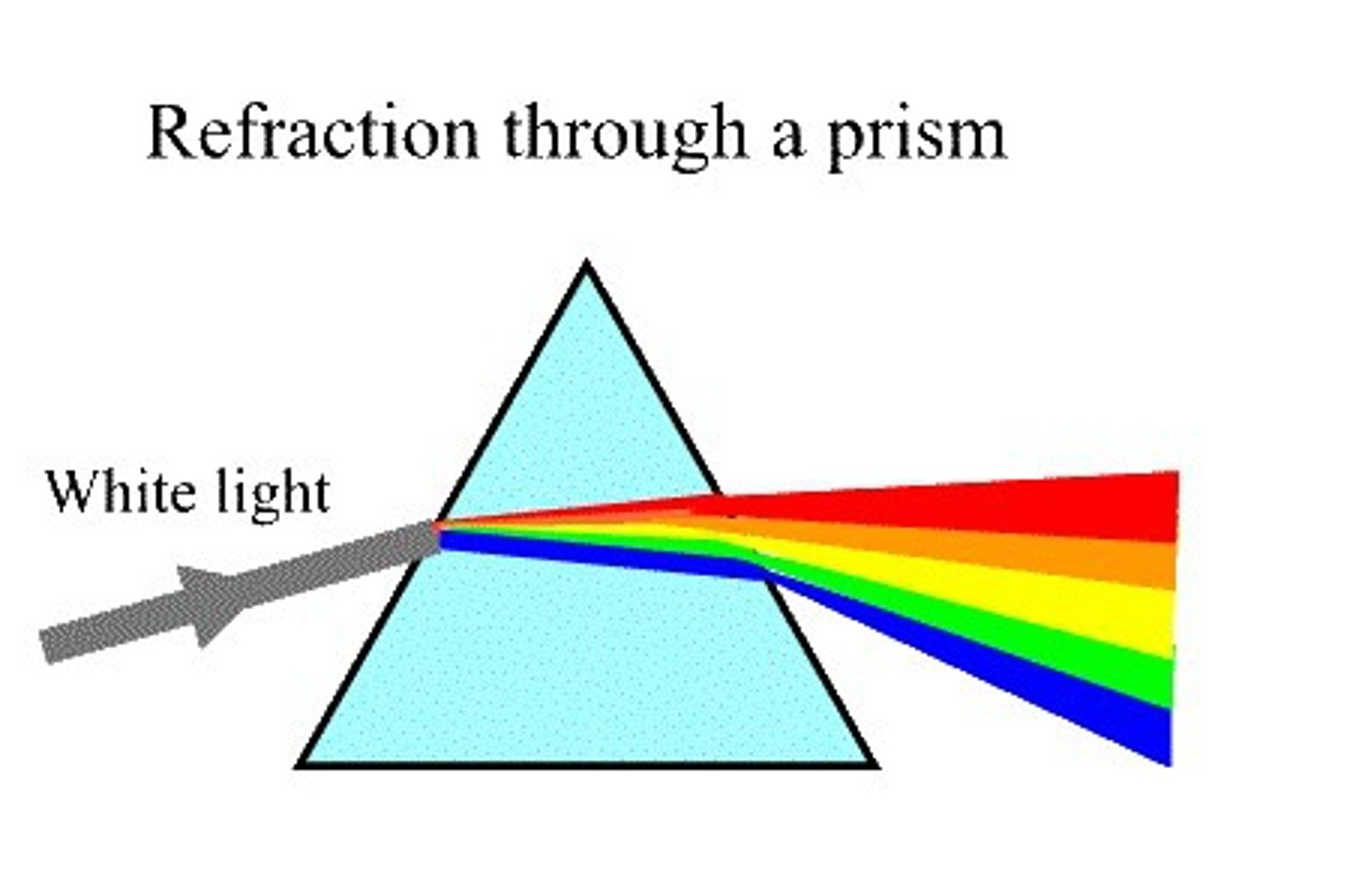
refraction
light changes velocities when traveling in air vs. water (objects in water appear to bend)

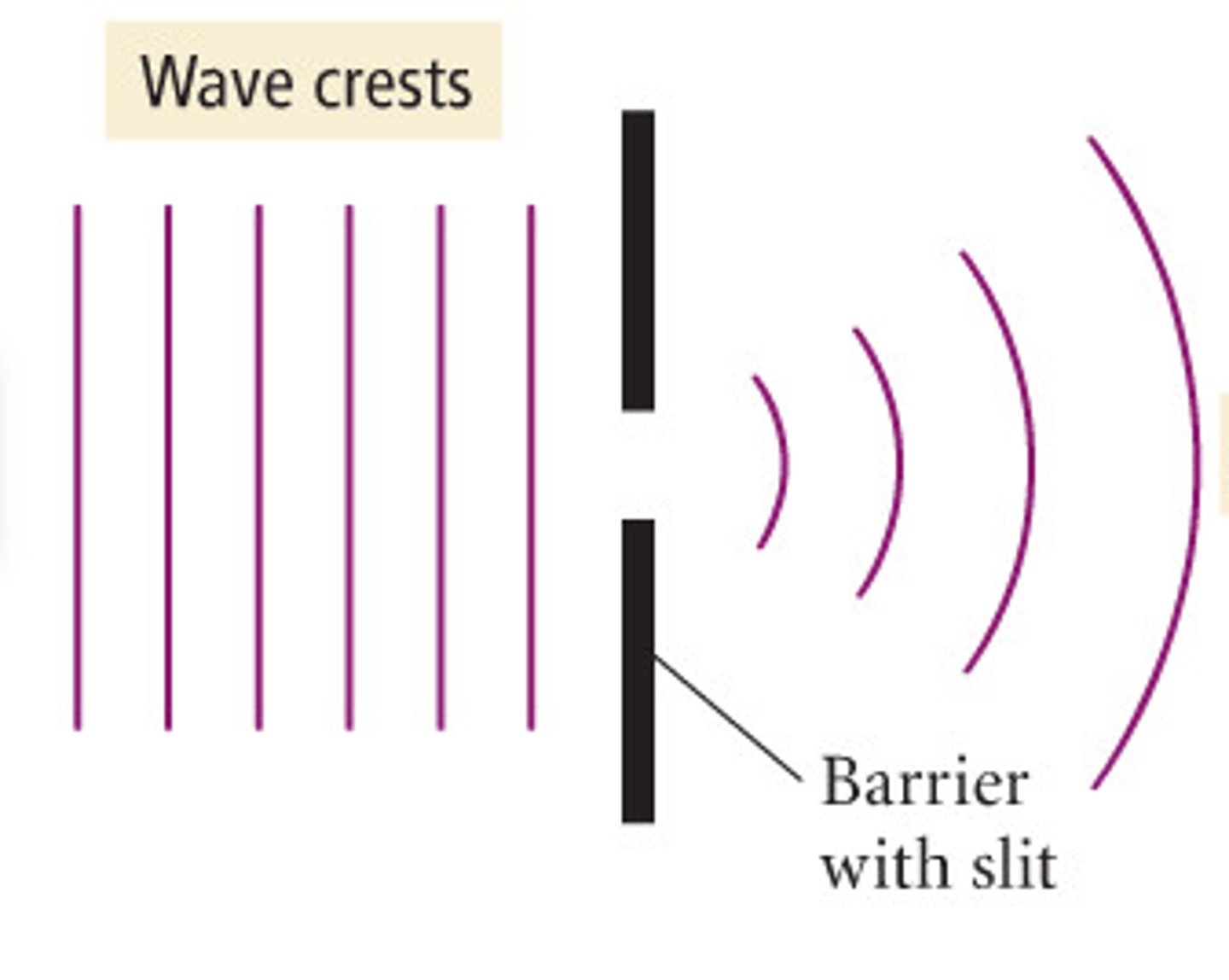
diffraction
when light strikes edge of object, it bends around it
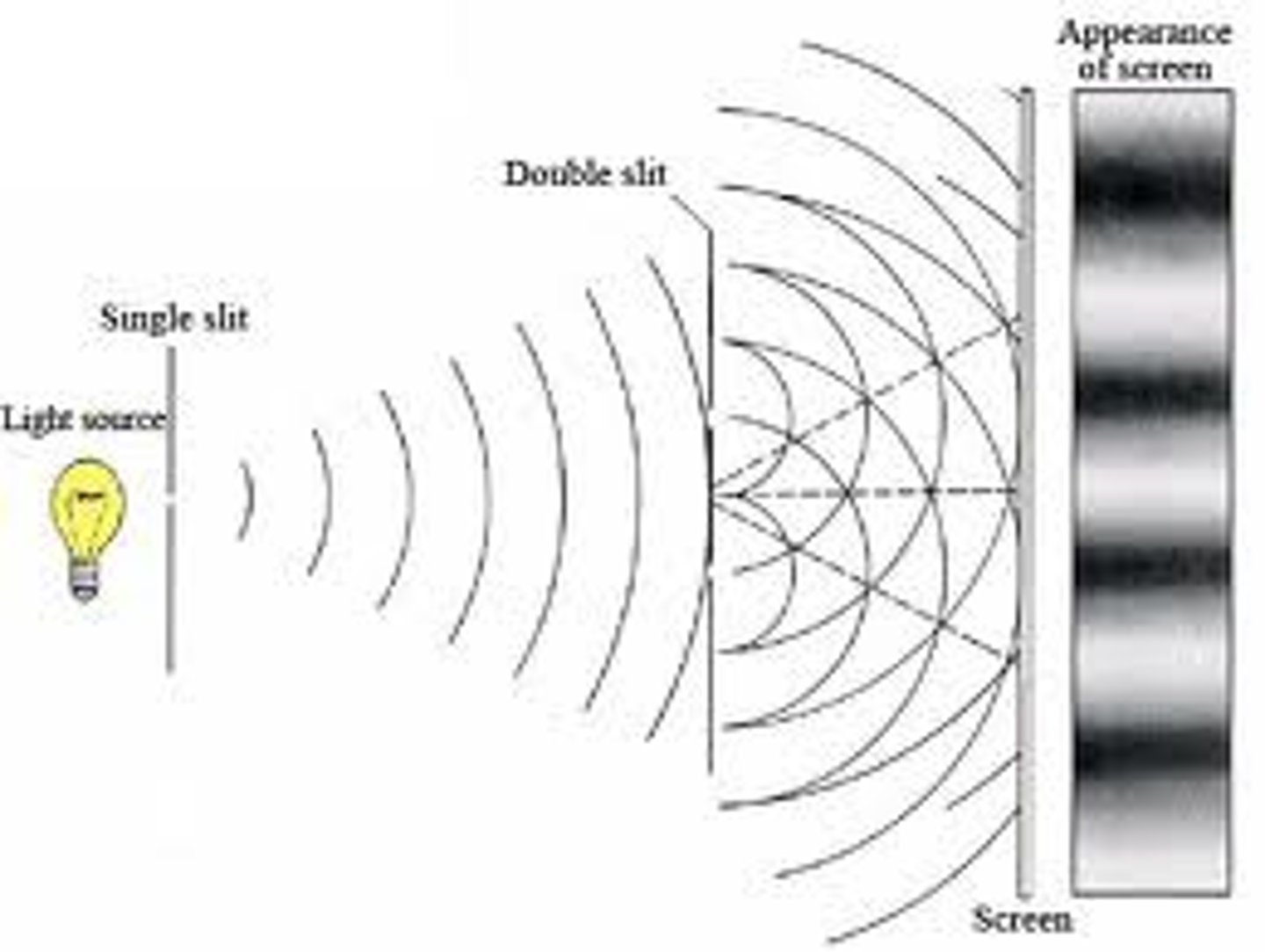
Young's double slit experiment
shows the constructive and destructive interference of waves that occur as light passes through parallel slits, resulting in dark spots from out-of-phase waves destructively interfering (nodes) and light spots from in-phase waves constructively interfering
electromagnetic radiation
a form of energy that exhibits wavelike behavior as it travels through space
electromagnetic spectrum trends
long wavelength = low frequency & low energy (radio waves)
short wavelength = high frequency & high energy (gamma rays)
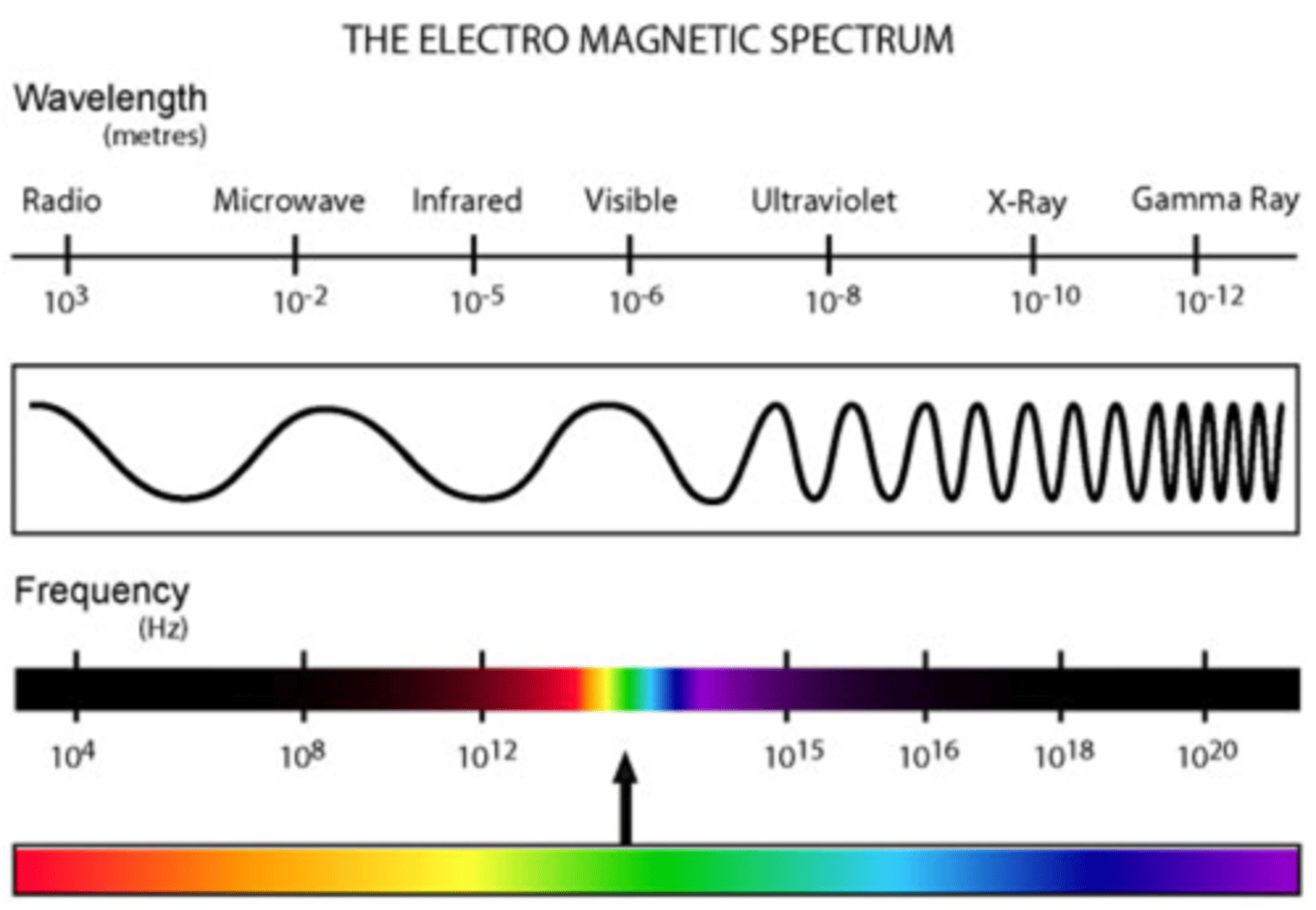
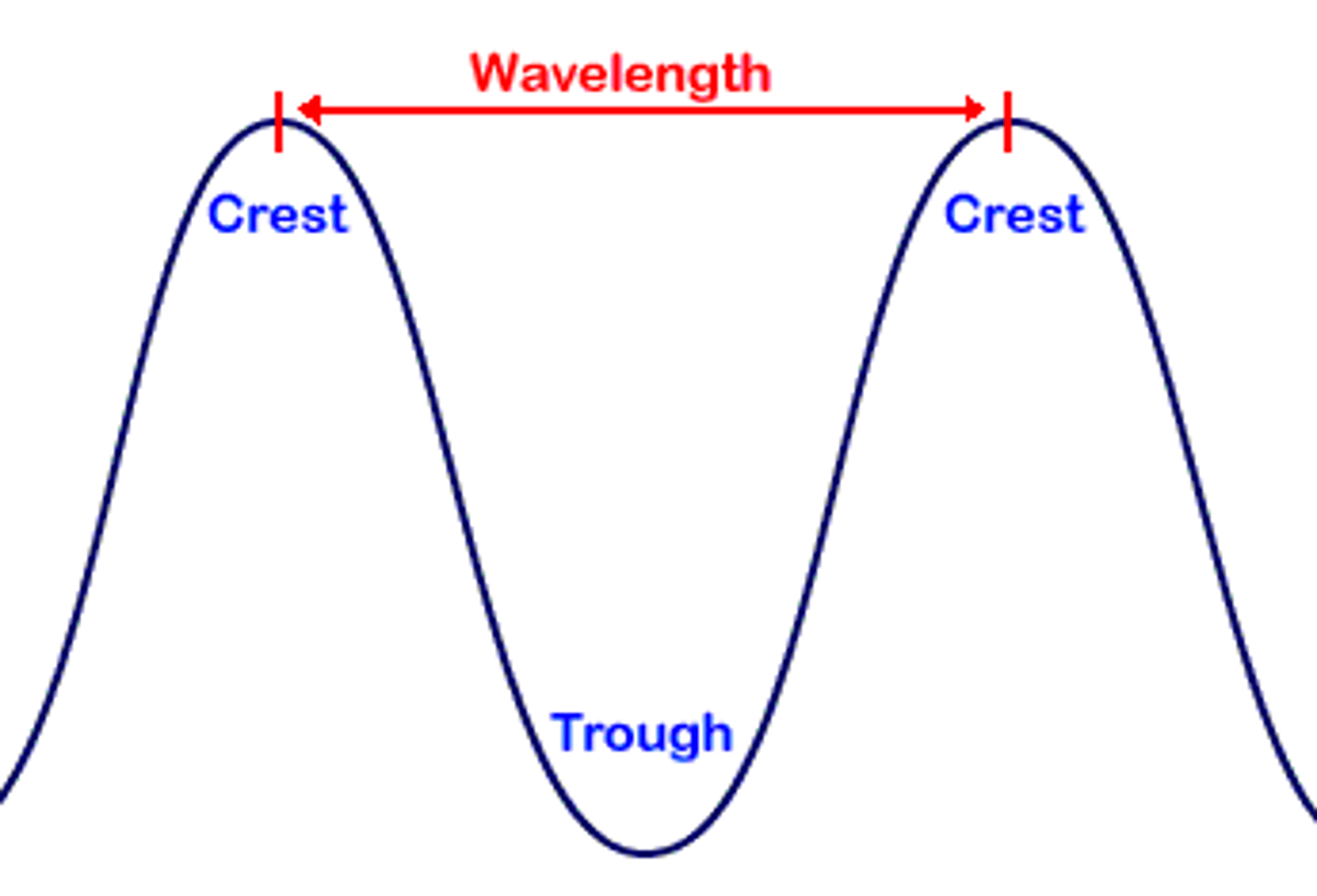
wavelength
(lambduh) horizontal distance between the crests or troughs of two adjacent waves
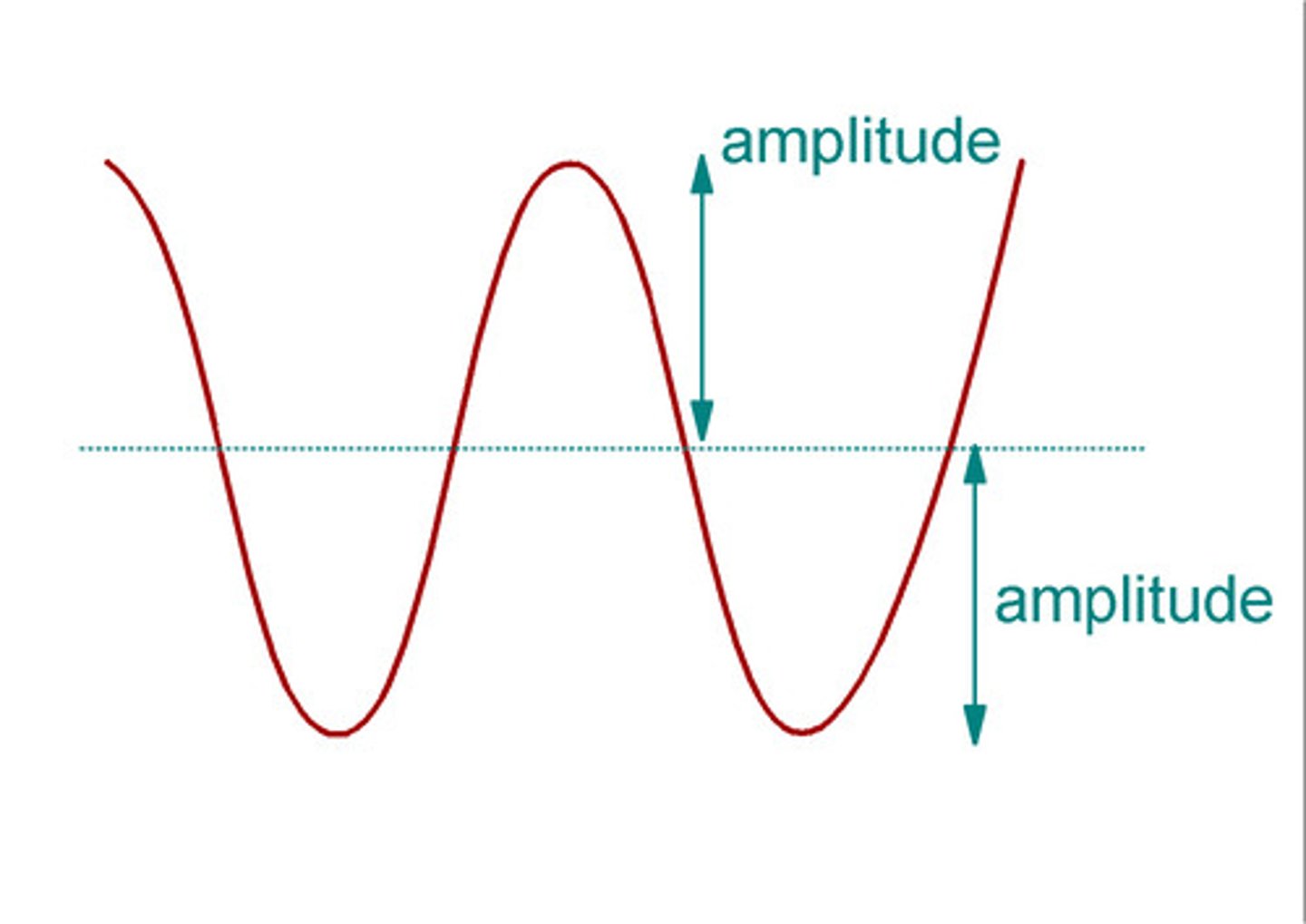
amplitude
height of wave (determines intensity/brightness)
frequency
(v = "nu") # of cycles per second / how often signal is detected per unit of time
frequency and wavelength relationship
inversely related (lower frequency = higher wavelength)
c= lambduh * frequency
the quantum concept: blackbody radiation
coals in campfire glow red and finish at blue/white, proceeding from long to short wavelength on EM spectrum
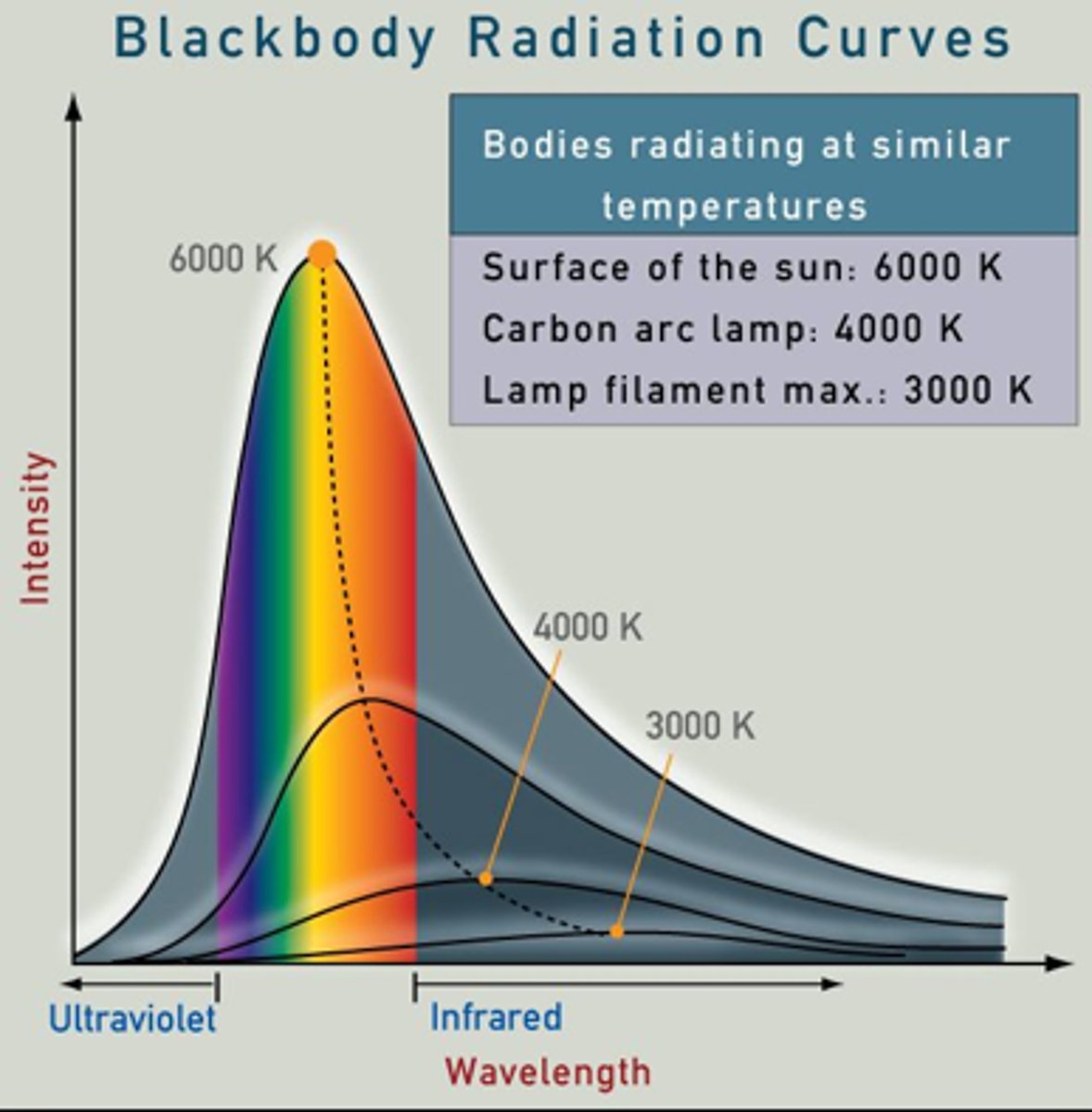
quanta
discrete packets of energy (exchange between matter and energy occurs in quanta)
energy and frequency relationship
directly proportional
E=nhv
n = # of photons
v = frequency
h = Planck's constant
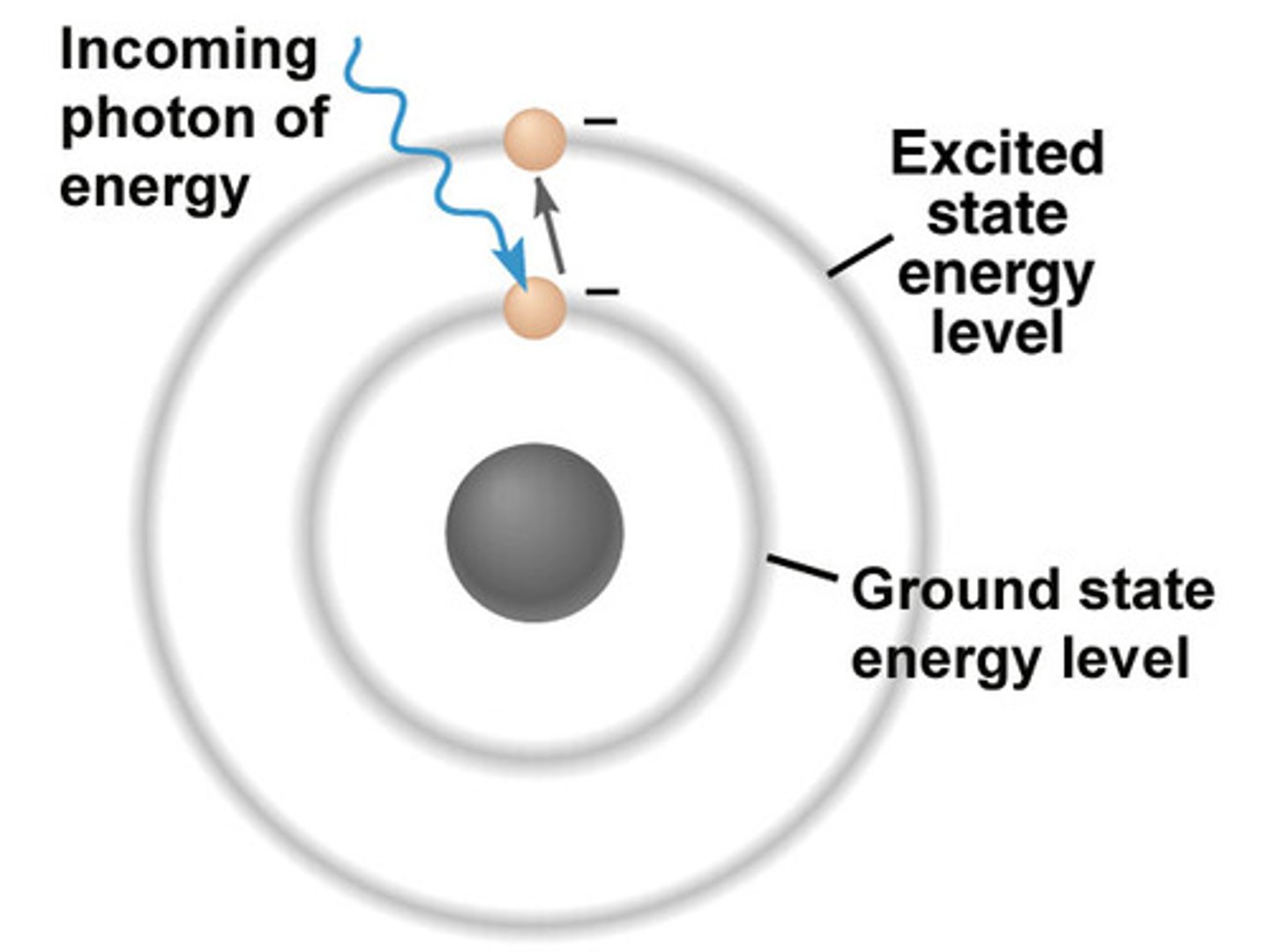
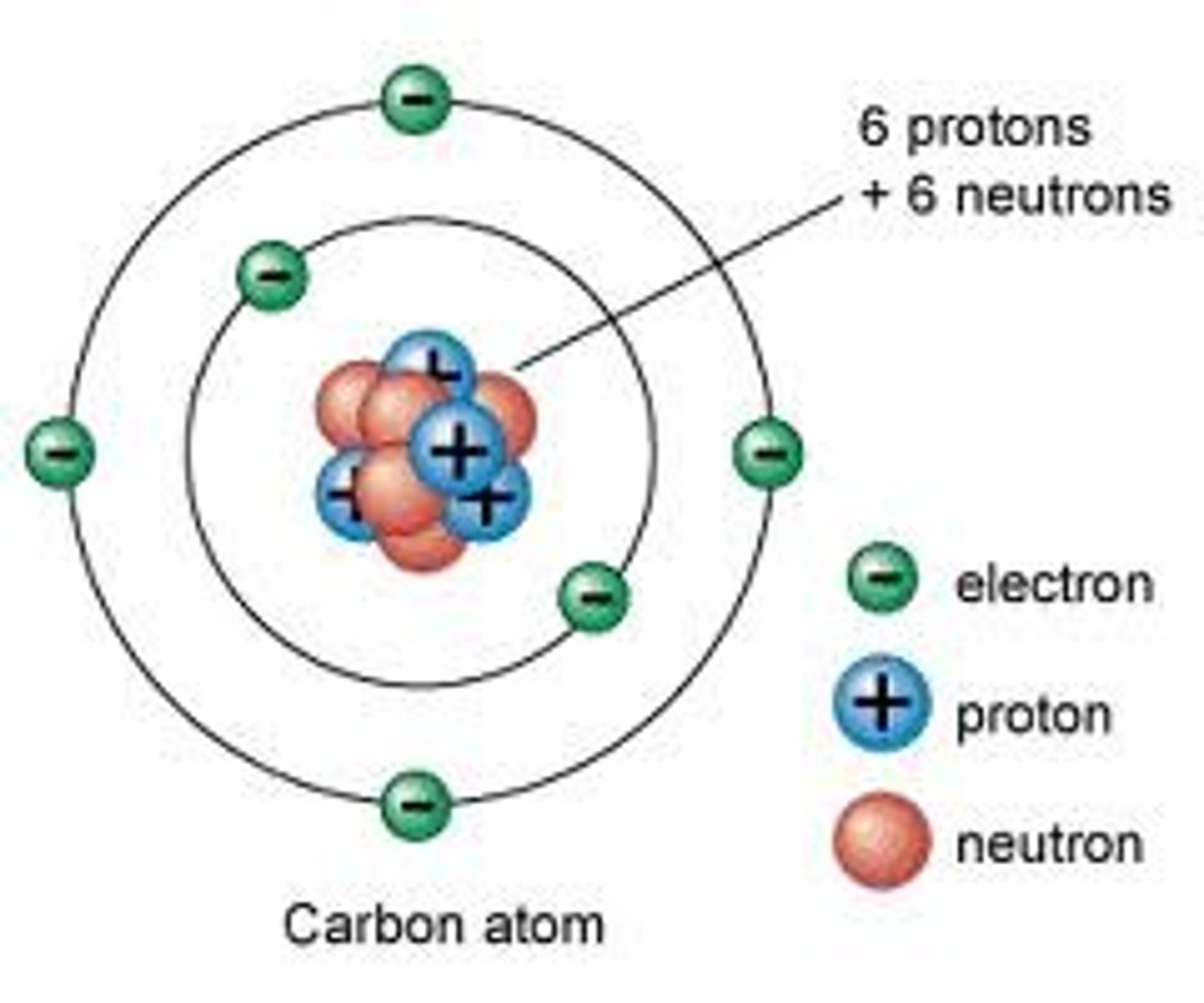
Bohr model of the atom
-electrons move in circular orbits about the nucleus
-electronic motion described by classical physics (planets around sun)
-electron has fixed set of orbits called stationary states
-as long as the electron remains in a given orbit, its energy is constant
-energy is neither emitted nor absorbed if electron remains in orbit
-an electron can pass only from one allowed orbit to another
-electron transitions result in light absorption and emission
-this light is rendered as discrete bundles of energy, called quanta
-caused shift to quantum mechanics
problems w/ the Bohr Model
-Bohr Model is obsolete in 21st century
-inability to explain multi-electron systems
-no explanation for magnetic field effects on atomic-emission spectra
-no basis for quantized angular momentum forcing electrons in orbits
-electron expected to spiral into nucleus due to attraction between positive nucleus & negative electrons
quantum theory
a model of matter and energy based on the principle that energy is absorbed or emitted in discrete packets (quanta)
quantized
-having values restricted to whole-number multiples of a specific base value (n is quantized)
-Planck proposed that objects emit EMR in integral numbers of quantum defined by E = hv
equation that relates energy of a quantum of electromagnetic radiation to its wavelength
E = hc / lambduh
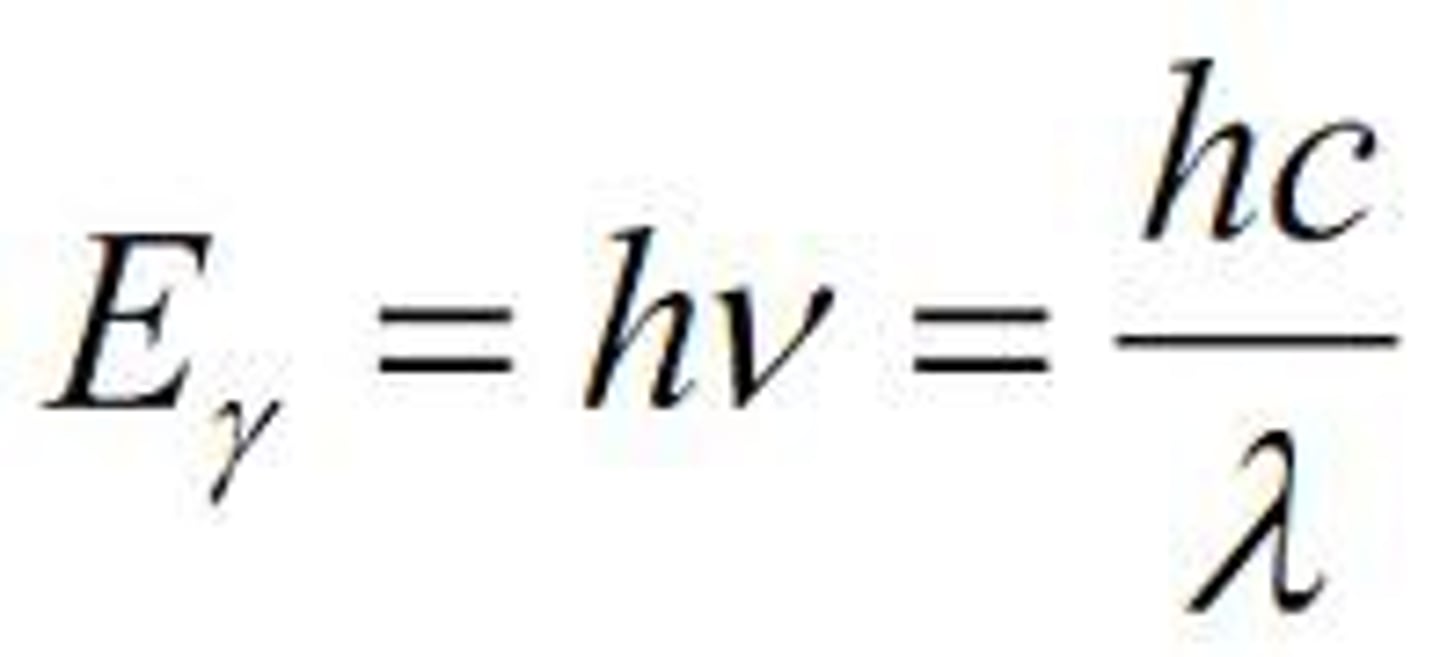
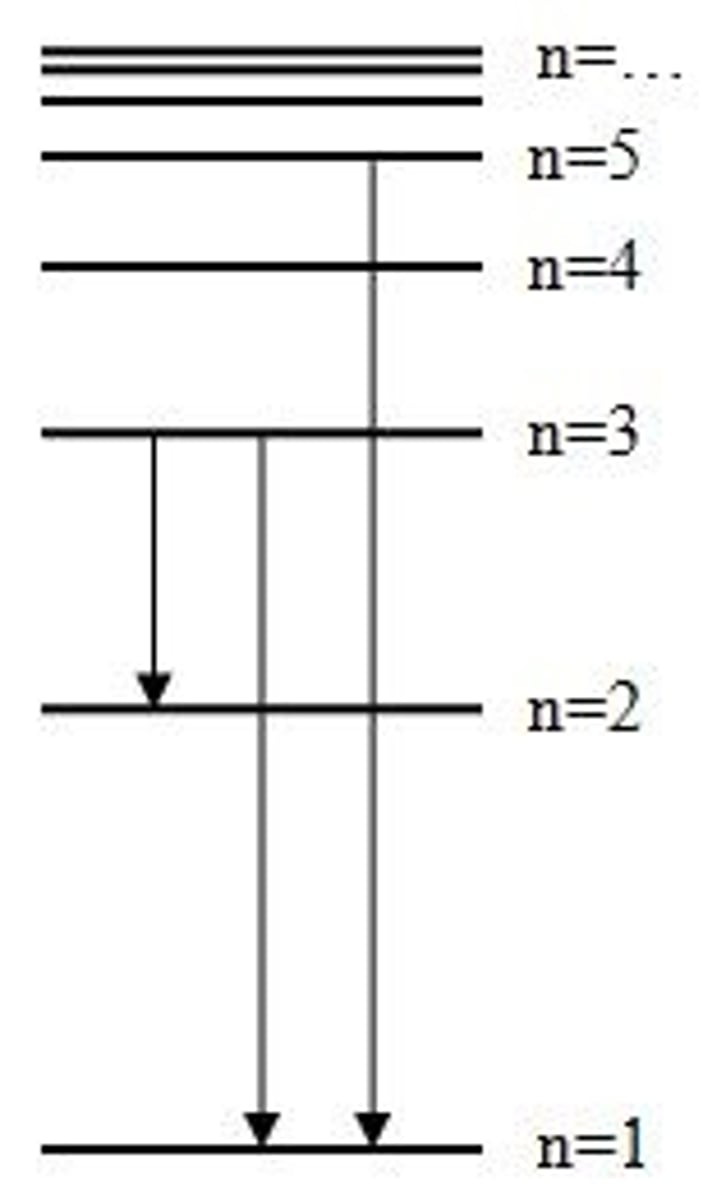
emission
release of light from excited state atoms (when electrons return to lower n, energy is released)
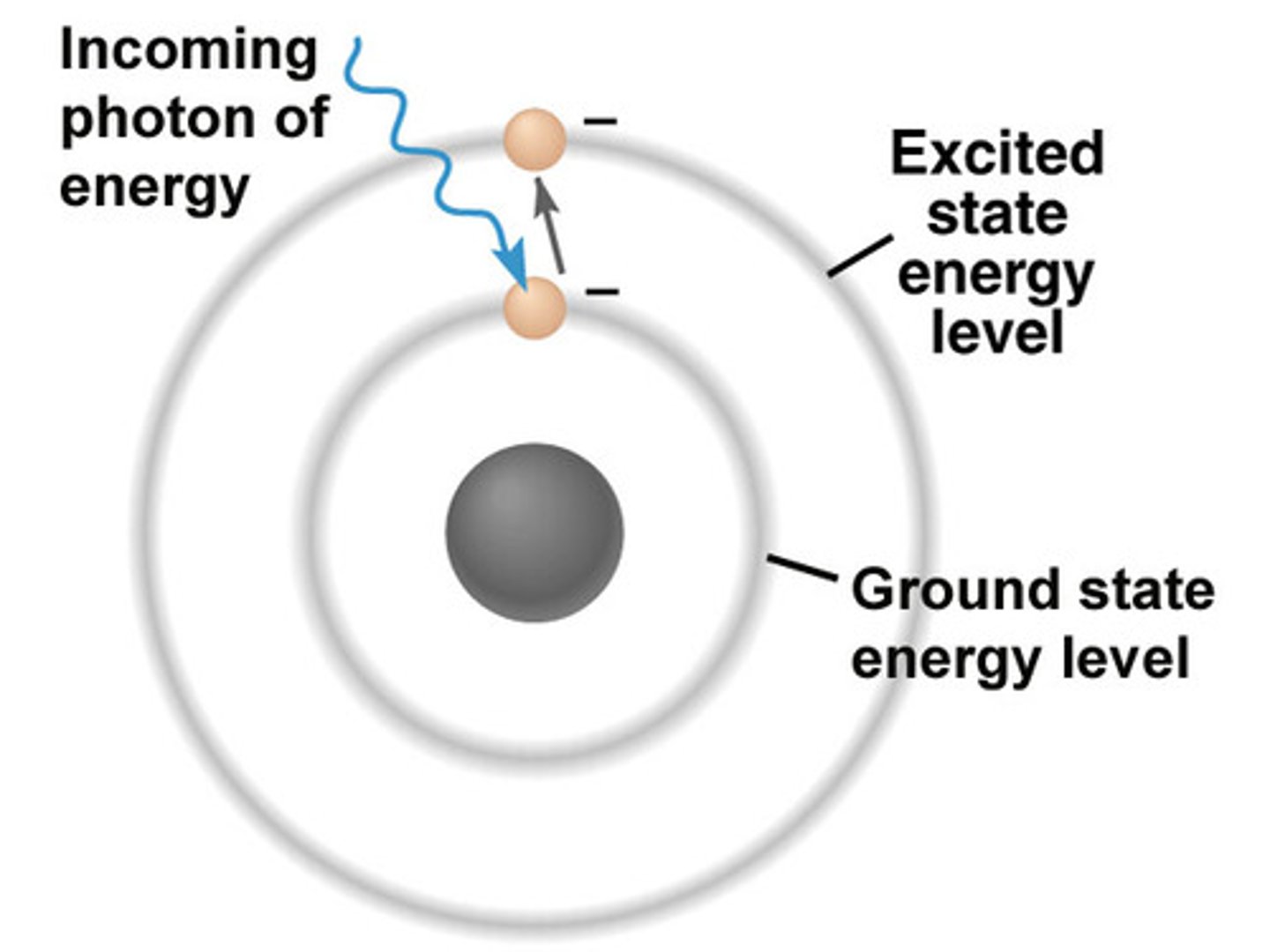
absorption
promotes electron to higher energy (excited state)