O Chem I - Functional Groups + pKa
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
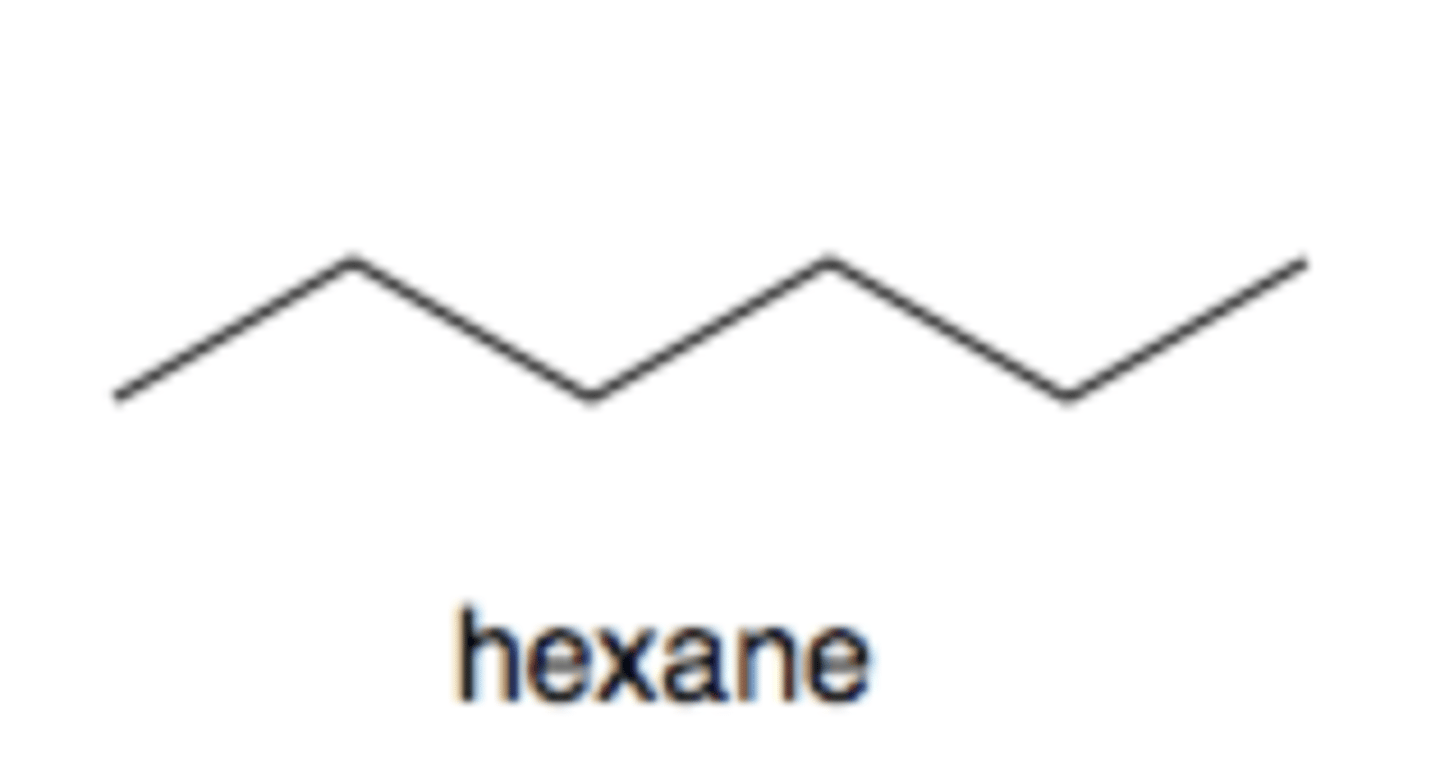
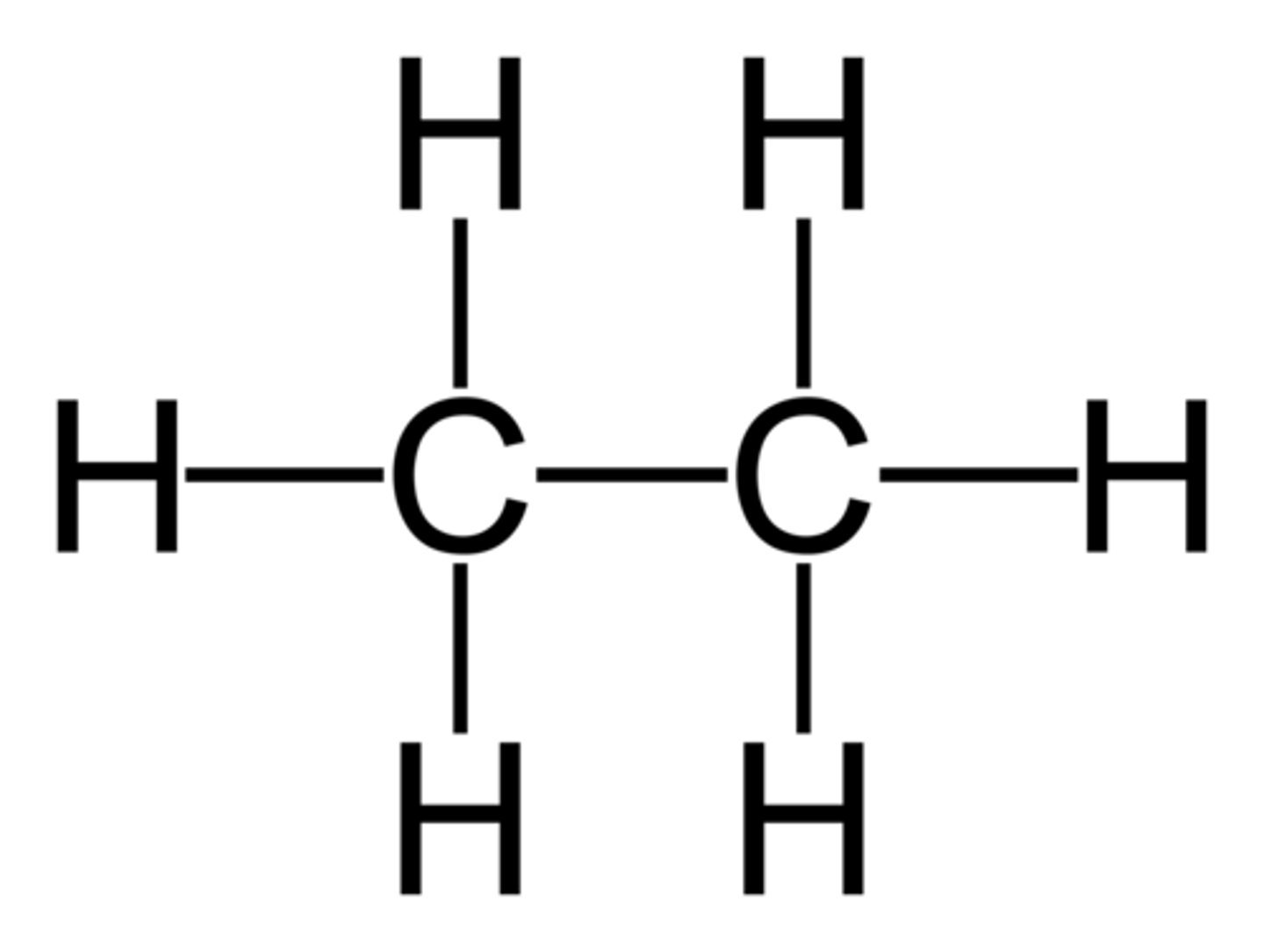
Alkane
C-C and C-H single bonds no rings
* not technically a fxnl group *
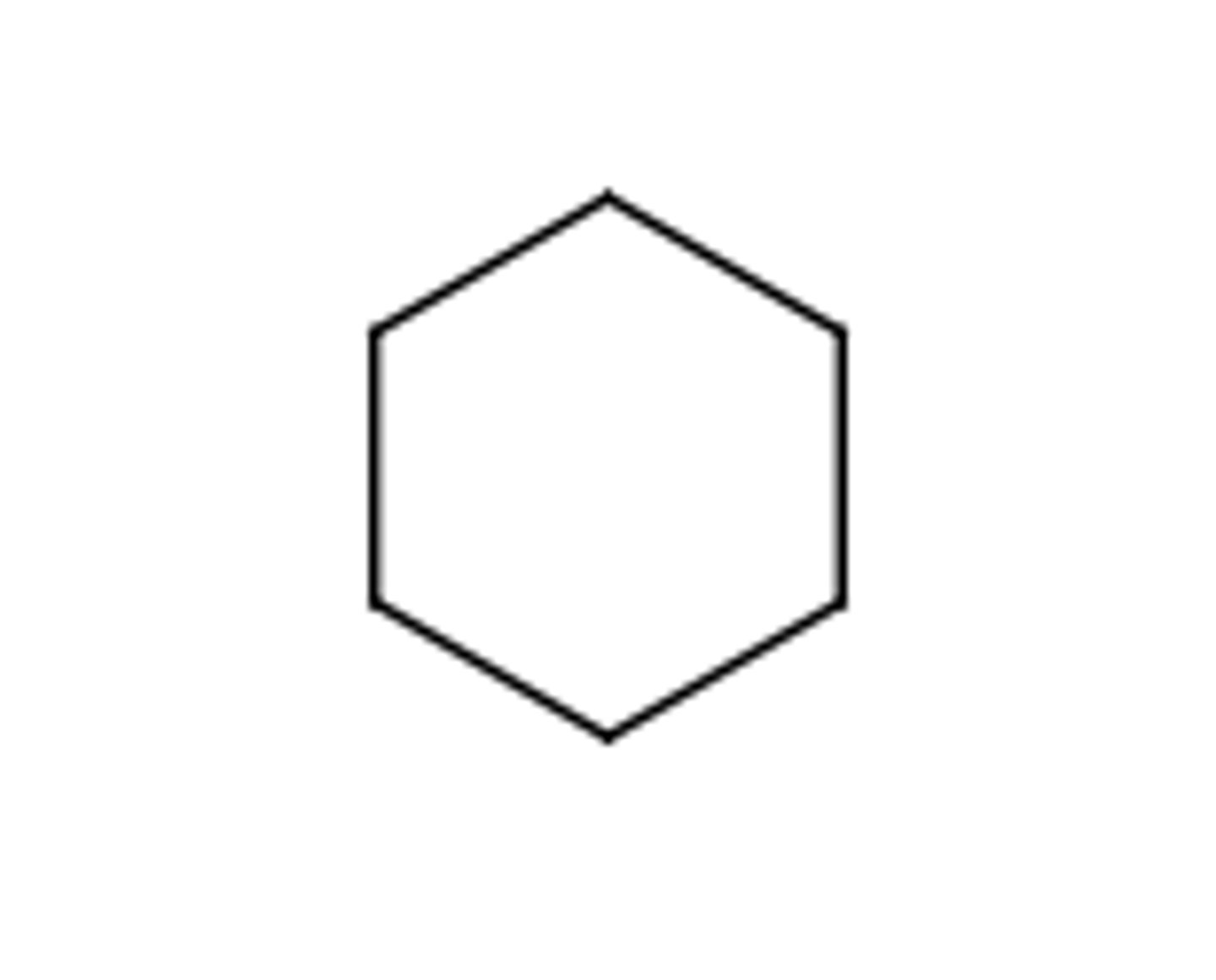
Cycloalkane
C-C and C-H single bonds WITH rings (cyclo)
* not technically a fxnl group *
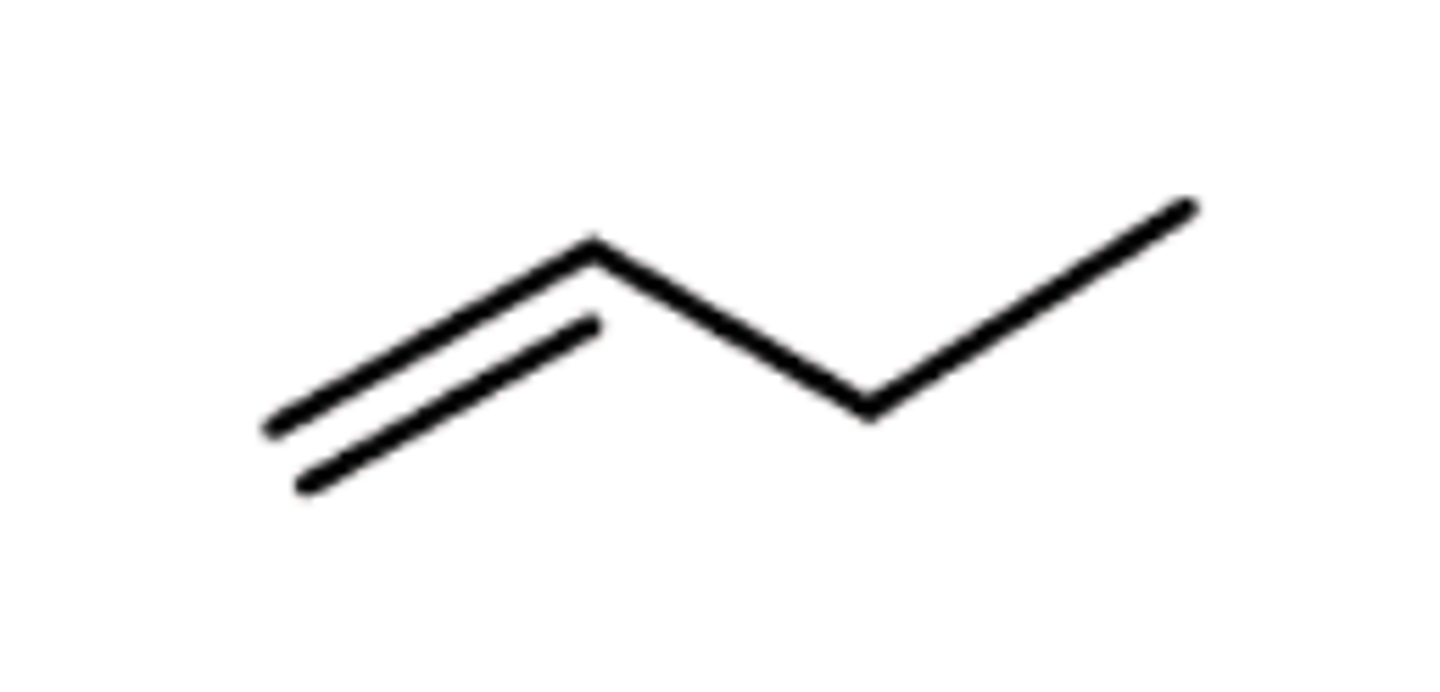
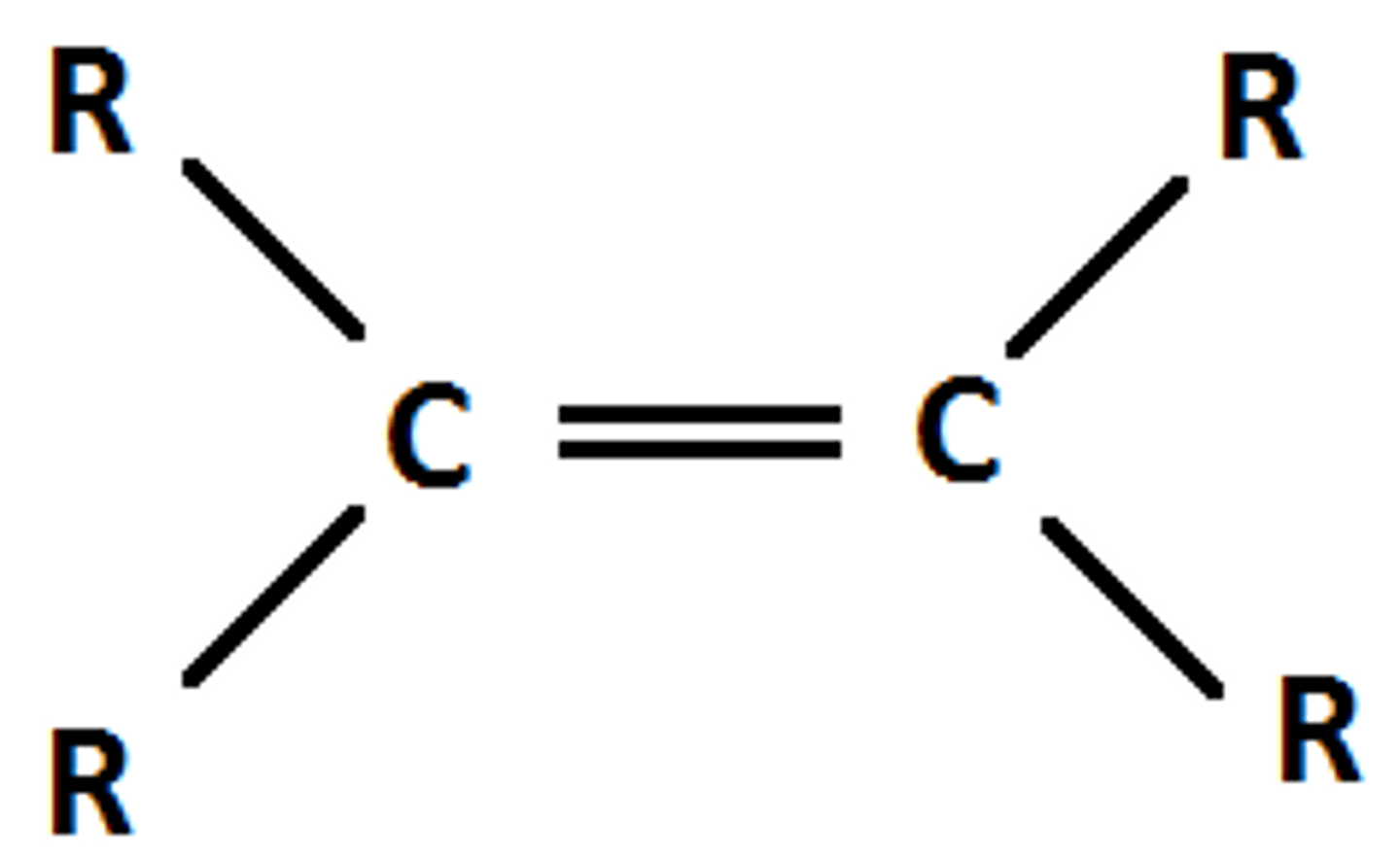
Alkene
C=C DOUBLE bonds
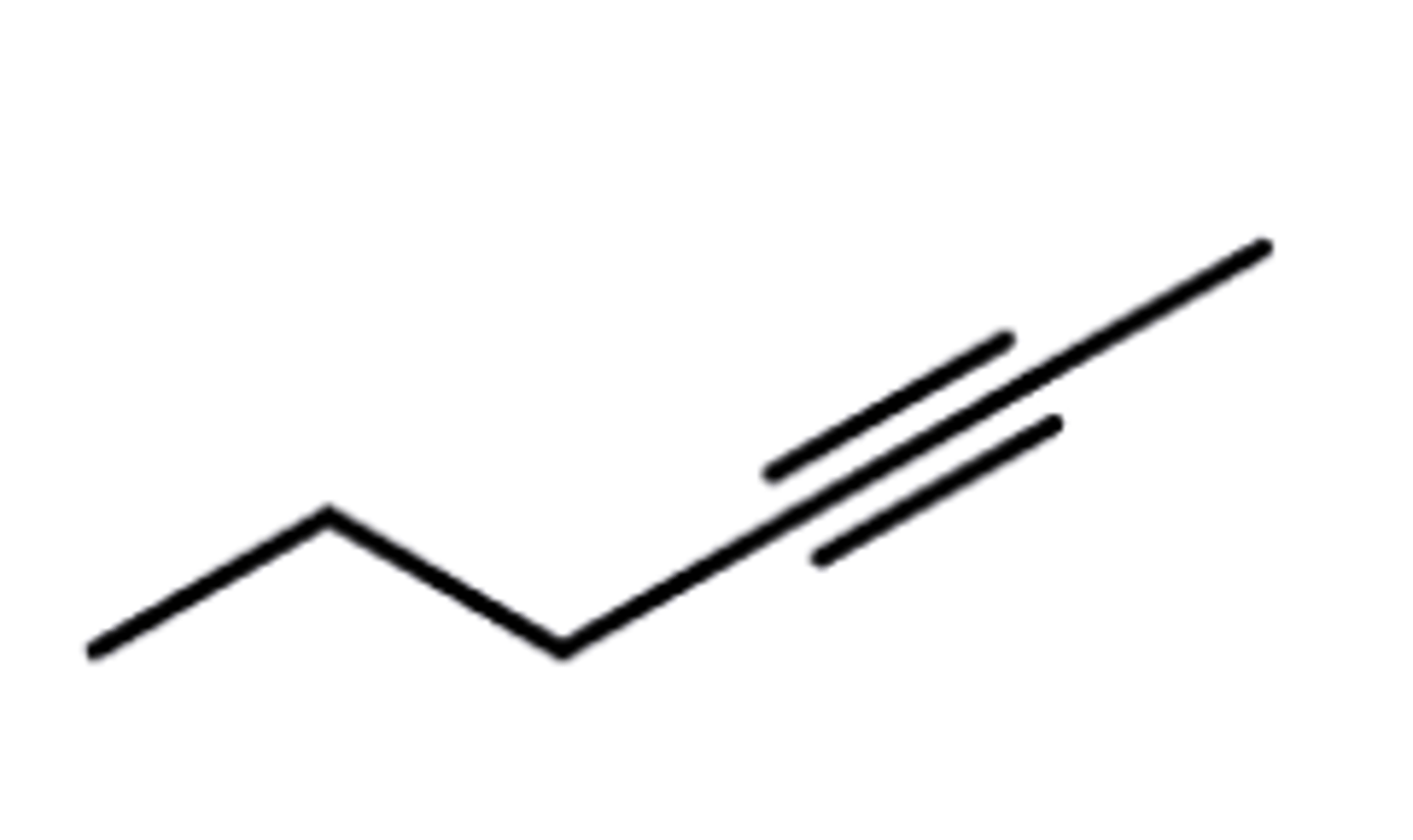
Alkyne
C+C TRIPLE bonds
(Internal --> additional carbon)
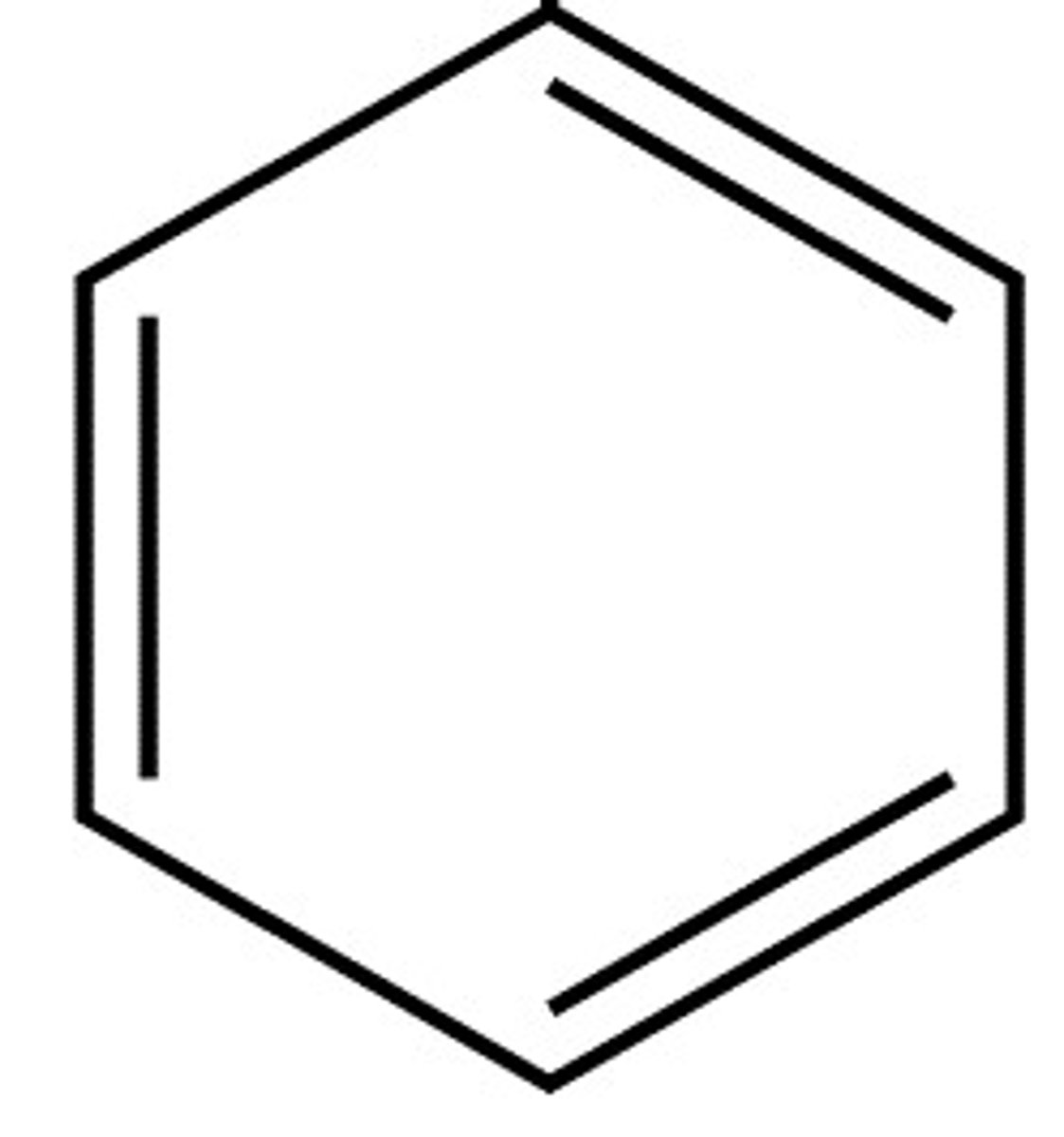
Arene (Aryl, Aromatic)
Odd # of C=C bonds --> "a" RING
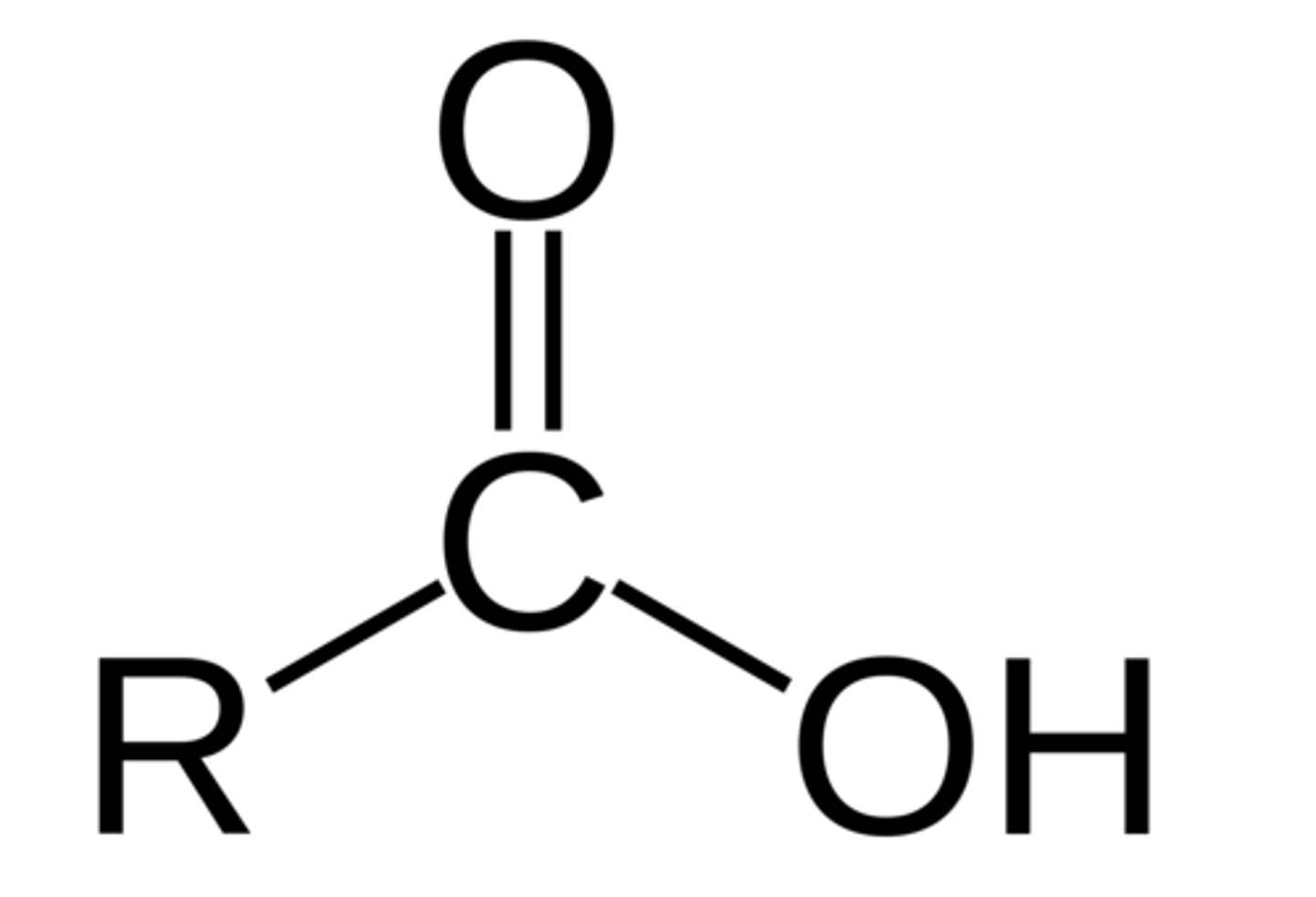
Carboxyl Group
COO-H
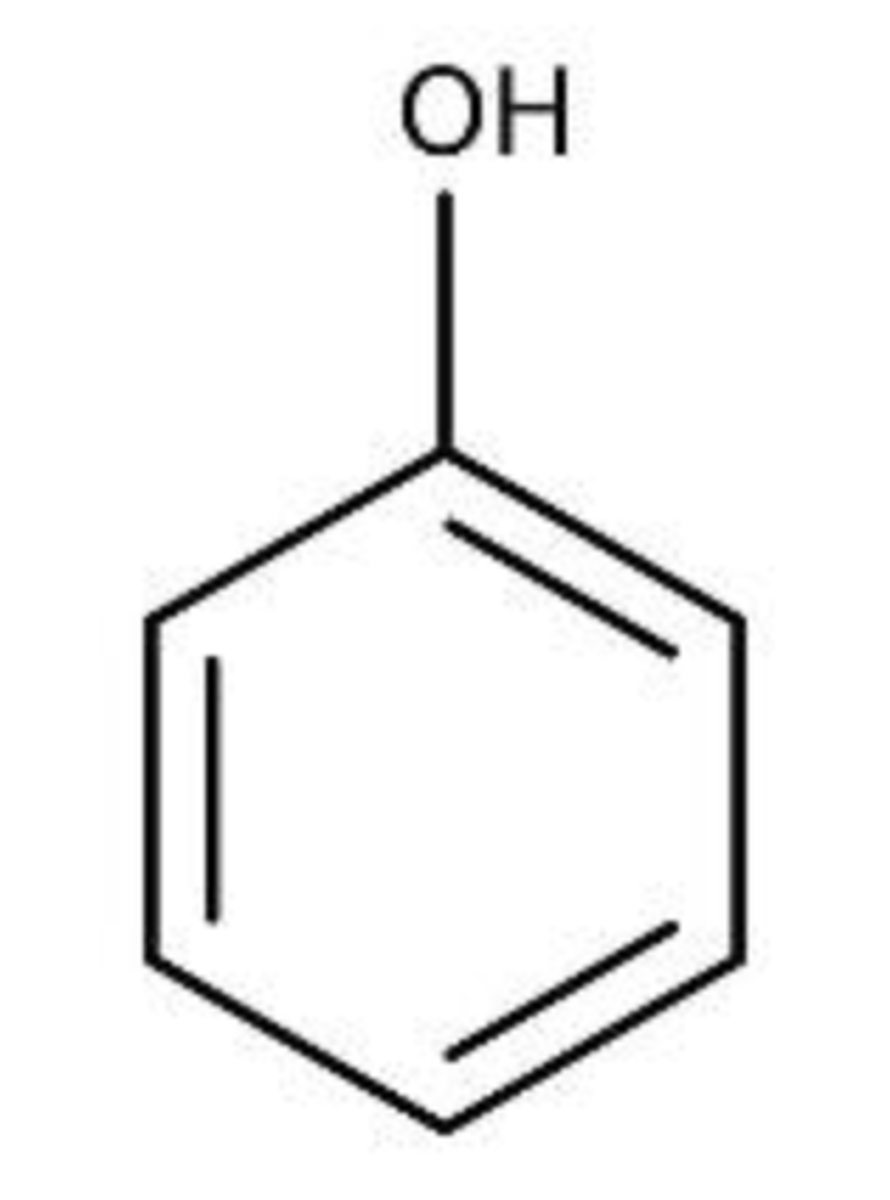
Phenol Group
Ring + -OH (hydroxy group)
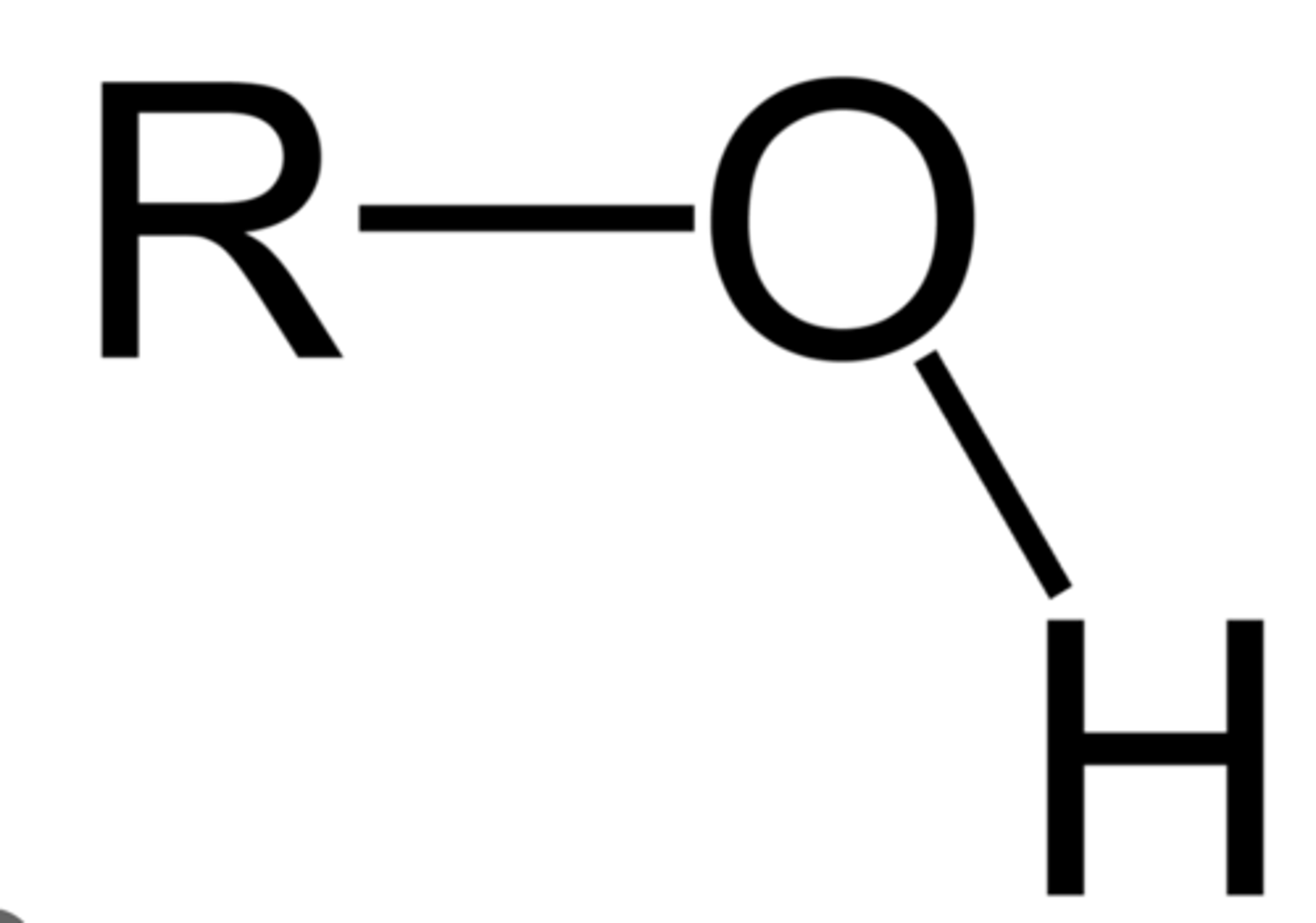
Alcohol
R-OH (hydroxy group) on a saturated carbon (no double bonds)
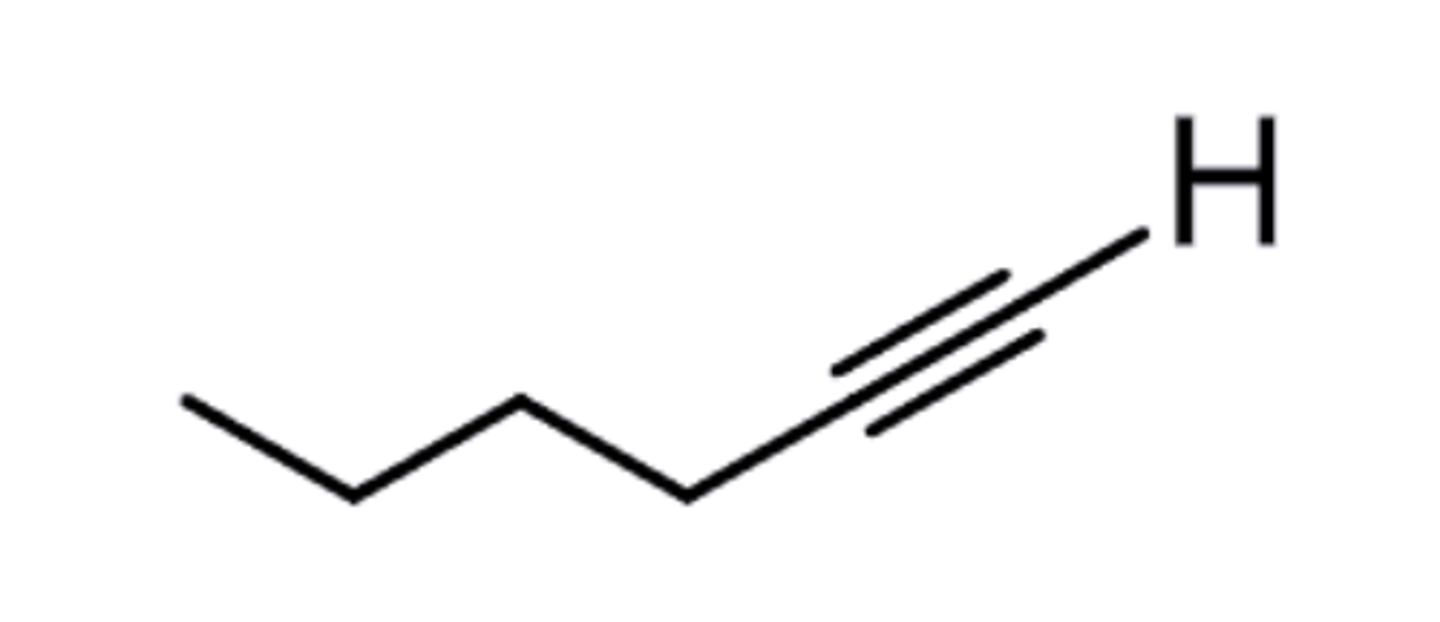
Terminal Alkyne
C+C triple bond is on the end of the strand - no other carbons just an H (terminal)
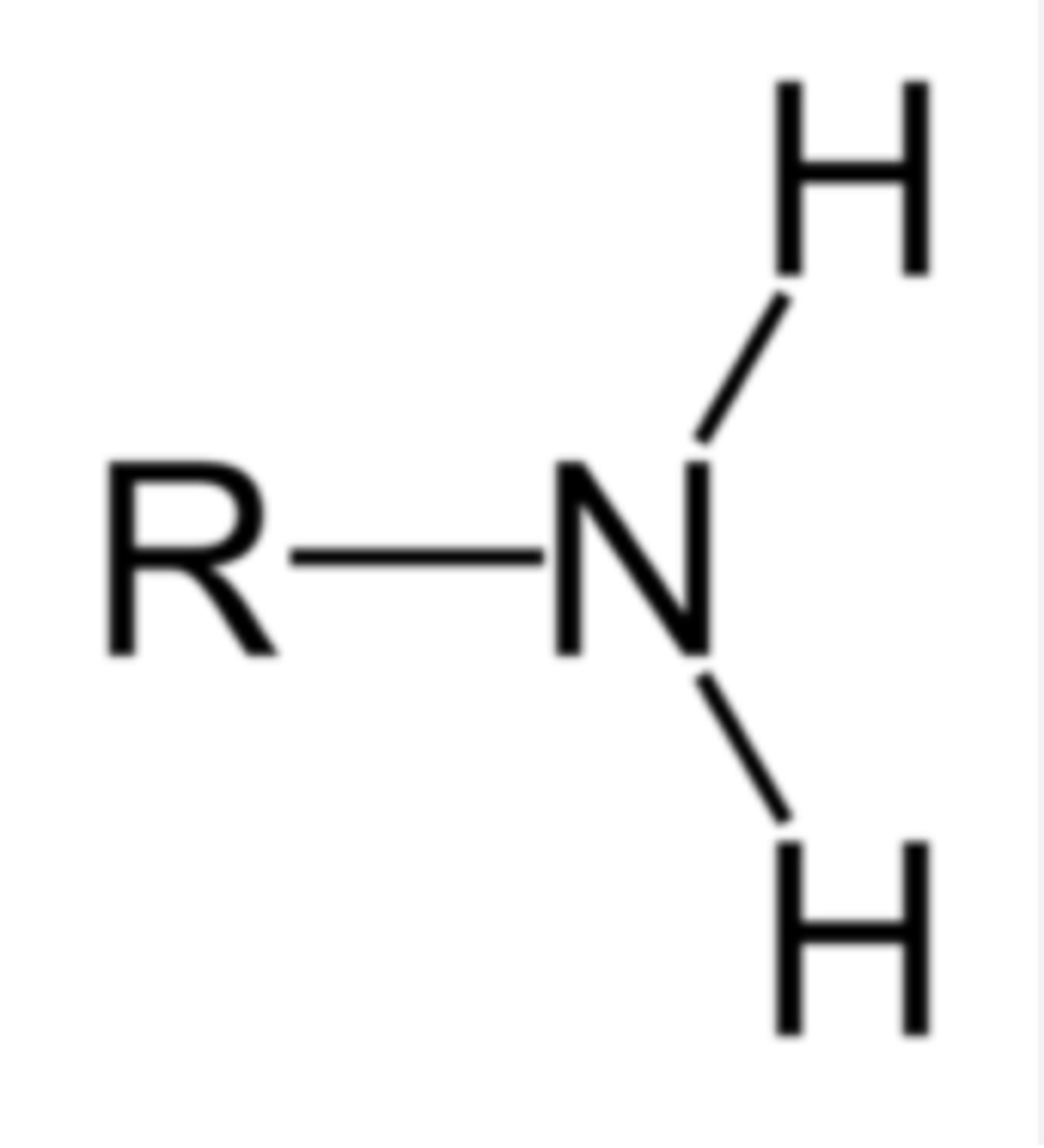
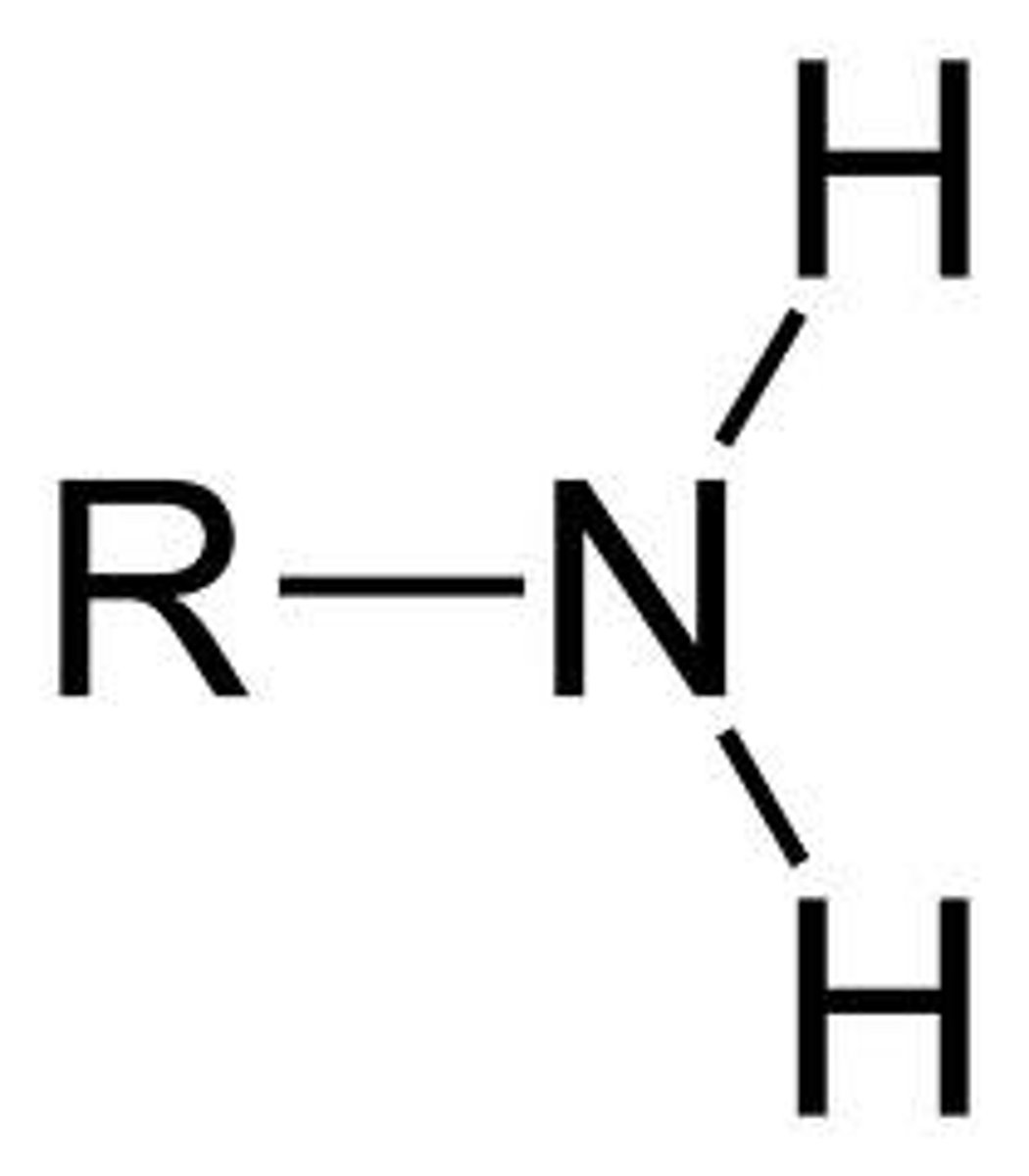
Amine
NH2 (amino group) - typically N has a lone pair of E-'s
H-Cl (Hydrochloric Acid) pKa?
Conjugate base?
-5 (STRONG mineral acid)
Cl- weak CB
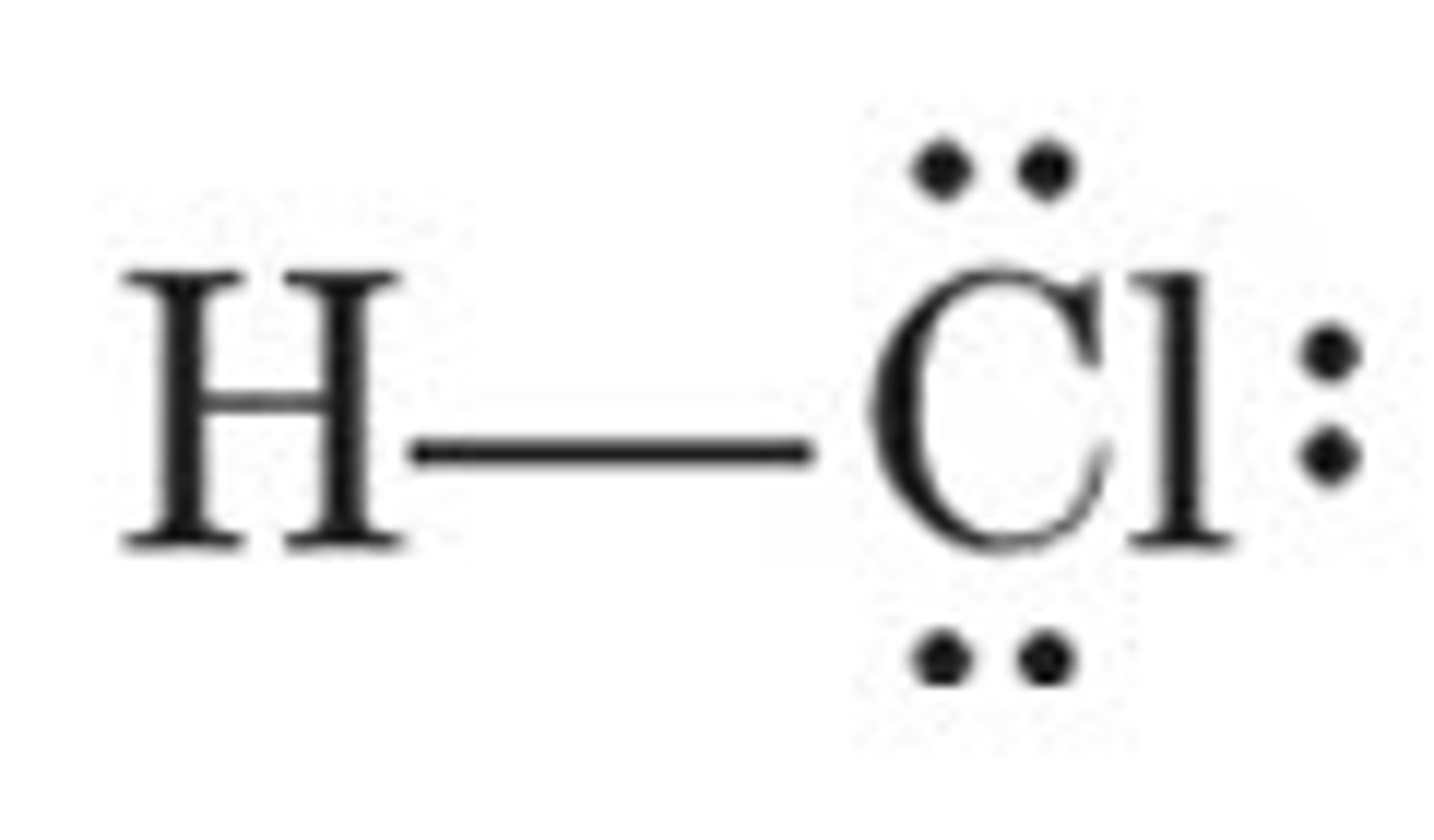
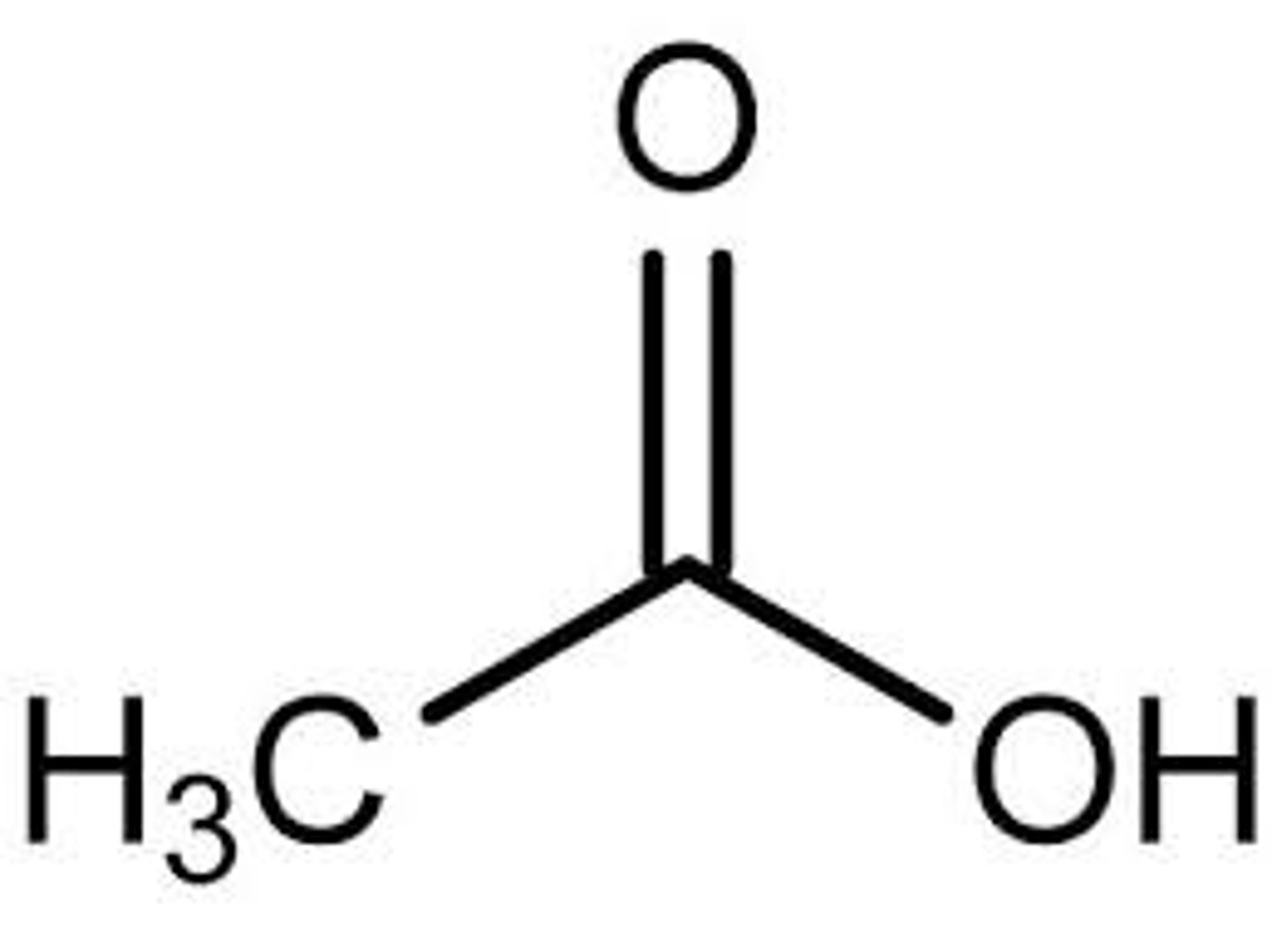
CH3COO-H (Carboxylic Acid) pKa?
Conjugate base?
5
CH3COO-
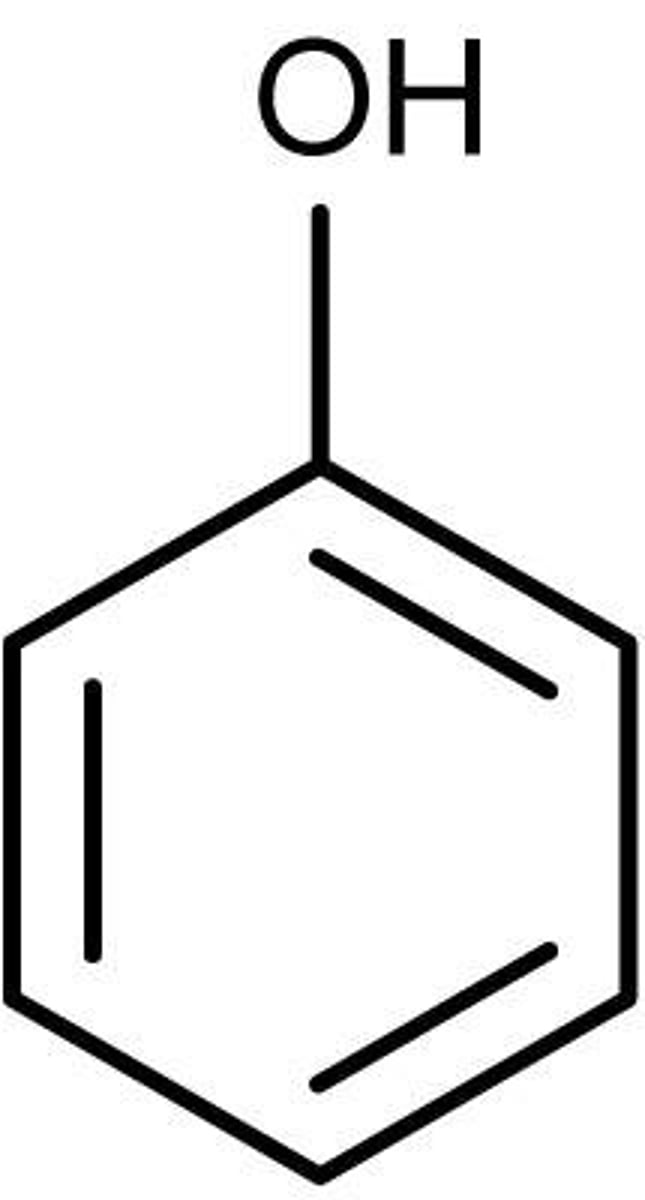
C6H5O-H (Phenol) pKa?
Conjugate base?
10
C6H5O-
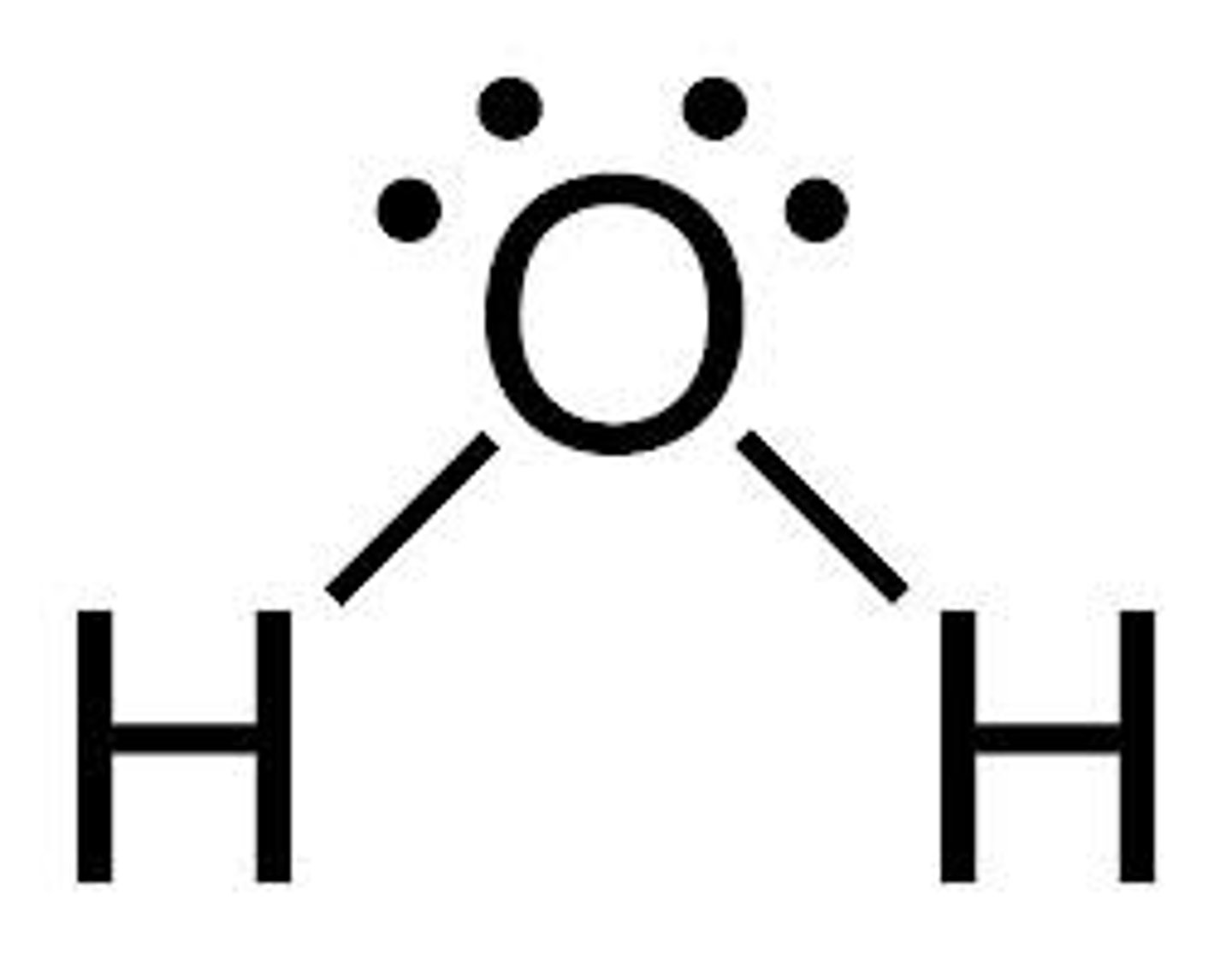
HO-H (Water) pKa?
Conjugate base?
15 (~ alcohol)
HO-
CH3CH2O-H (Alcohol) pKa?
Conjugate base?
15 (~~water)
CH3CH2O-
HC+C-H (Terminal Akyne) pKa? (+ = triple bond)
Conjugate base?
25
HC+C-
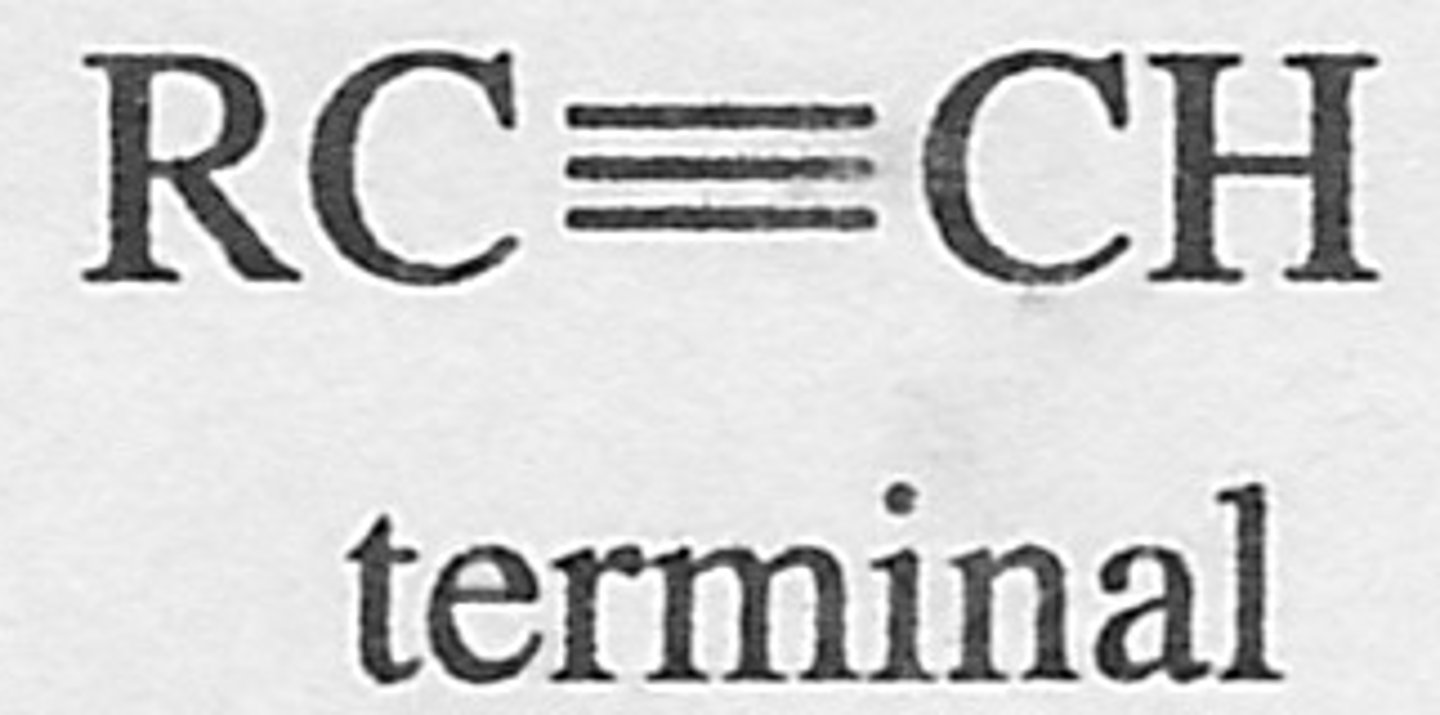
H2N-H (Amine) pKa?
Conjugate base?
40
H2N-
CH2=CH-H (Alkene) pKa?
Conjugate base?
45
CH2=CH-
CH3-H (Alkane) pKa?
Conjugate base?
50 (weakest acid)
-CH3 (strongest conjugate base)
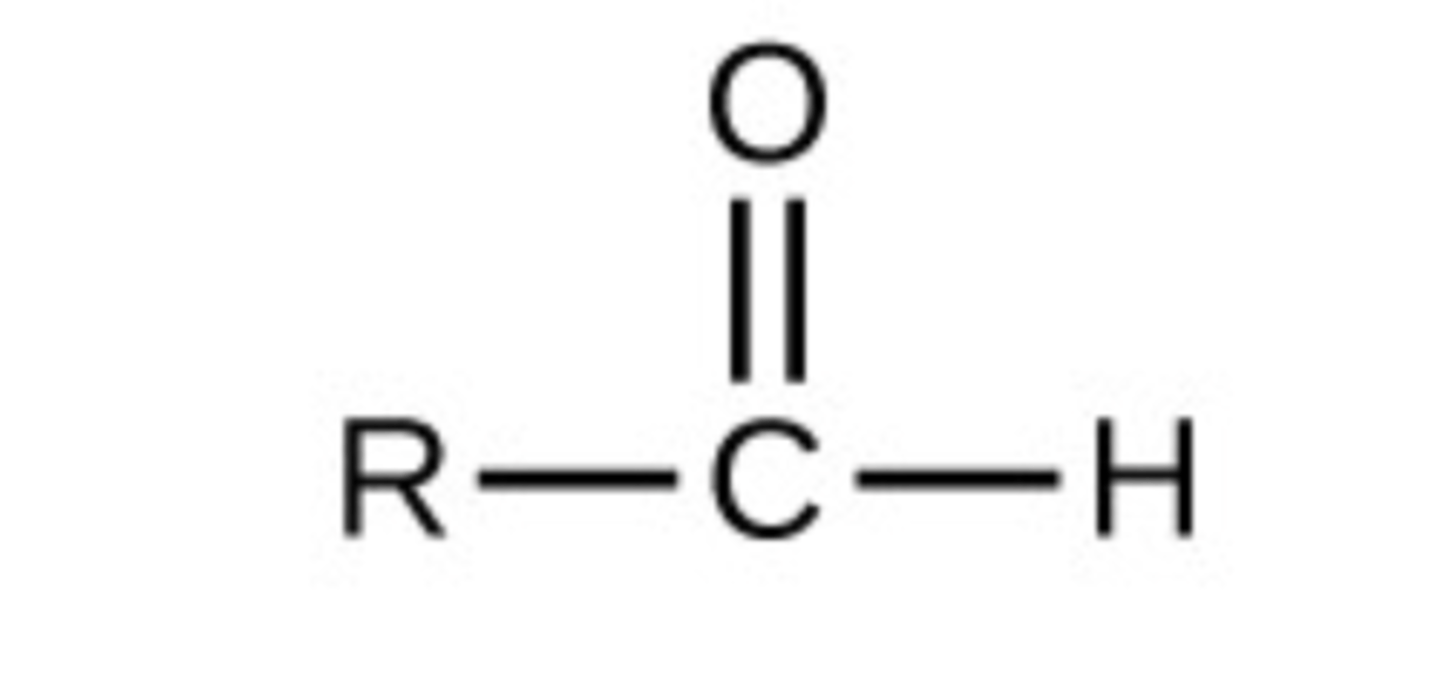
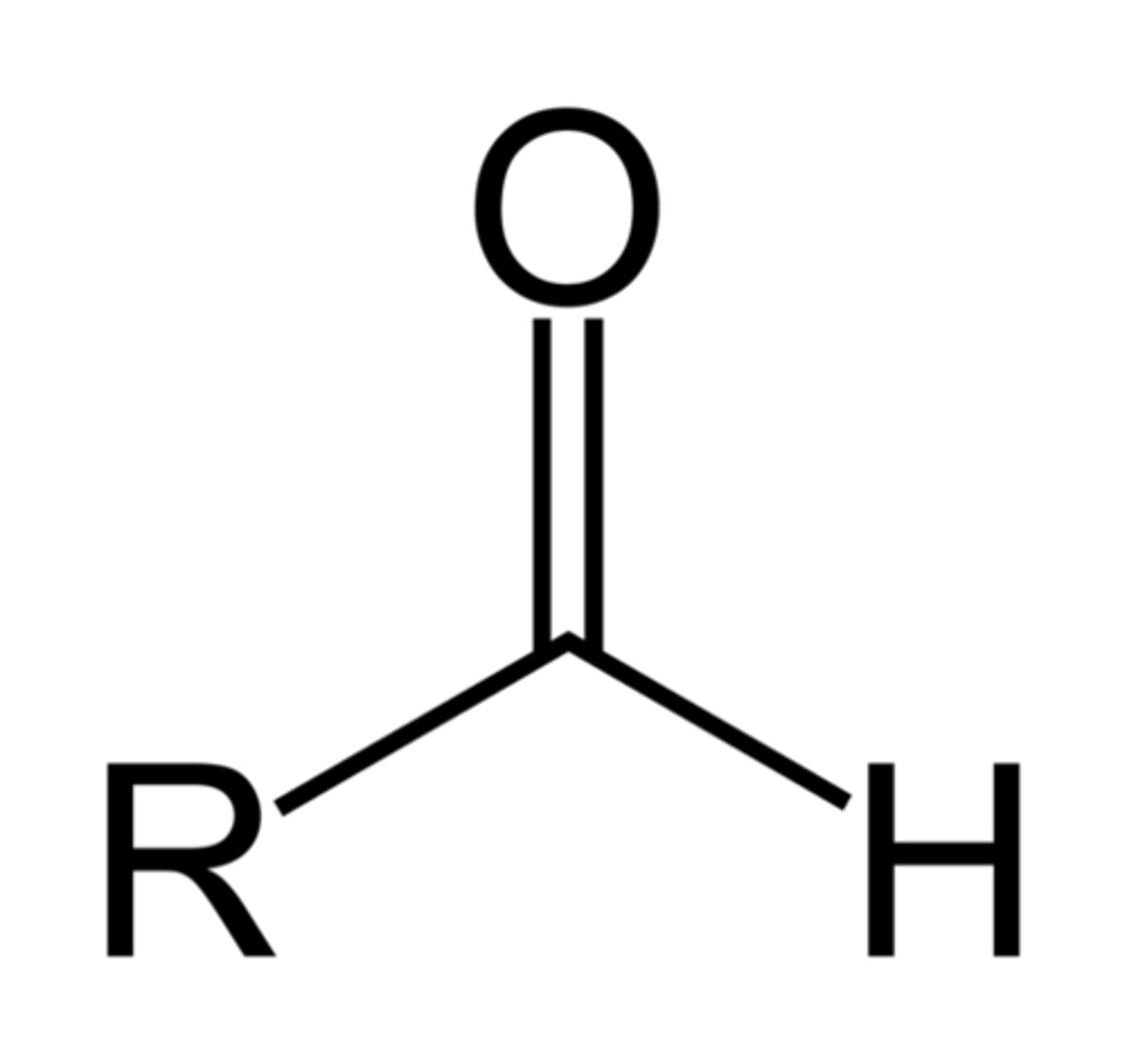
Aldehydes
R-CHO