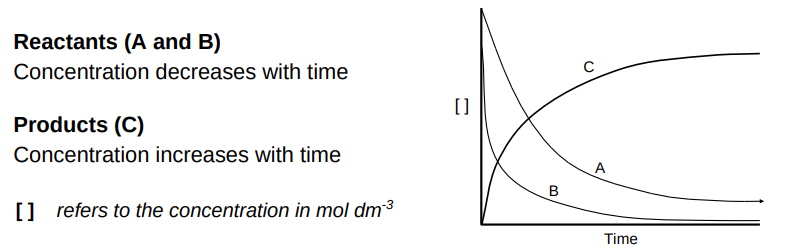Simple collision theory
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
Define rate of reaction and give its units
Rate of reaction - change in concentration of reactant or product / time
Measured in mol dm-3 s-1
Explain what happens to the rate of reaction throughout a reaction
Start of the reaction
Highest concentration of reactants
No products
Fastest rate of reaction due to most frequent collisions
As the reaction proceeds
Concentration of reactants has decreased
Concentration of products increases
Rate has slowed due to less frequent collisions
When the reaction stops
One of the two reactants has been used up (limiting reactant)
Highest concentration of products
Rate is 0
Explain how the collision theory affects rate of reaction (give the 2 conditions that must occur)
In order for a reaction to take place molecules must collide
Not all collisions lead to a reaction so certain conditions have to be met in order for a reaction to occur
Activation energy, EA
Molecules must have enough energy to overcome the EA
Orientation
Molecules must collide in the correct orientation
Explain how concentration affects rate
Higher concentration means more molecules in the same volumes
This leads to more frequent collisions
Faster rate of reaction
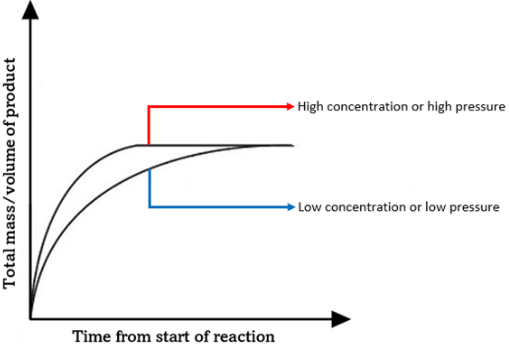
Explain the difference between the following on the rate of reaction, despite both equalling 0.04 moles:
20cm³ of 2.0 mol dm-3
10cm³ of 4.0 mol dm-3
4 mol dm-3 is a higher concentration than 2 mol dm-3:
4 mol dm-3 will have a faster rate of reaction (steeper line)
Both will create the same amount of product (line is level)
Explain how pressure affects rate
Same number of molecules in smaller volume
More frequent collisions
Faster rate of collisions
Explain how temperature affects rate
Increased temperature means more particles gain more kinetic energy to overcome energy barrier
Particles move more
More frequent collisions
More particles will now have energy greater than or equal to the activation energy
Explain how catalysts affect rate and how it does this
A catalyst speeds up the rate of chemical reaction without being used up
A catalyst lowers the EA by finding an alternative reaction pathway which has a lower EA

Draw a diagram showing the concentrations of the reaction
A + 2B → C