Cellular Respiration and Energy Metabolism Overview week 7
1/59
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
60 Terms
Cellular Respiration (why cells need energy)
Metabolism, Movement, Growth, Cell division, Action potentials,
cellular respiration
process of nutrient breakdown
with accompanying ATP synthesis
• Nutrients provide chemical energy
ATP
universal energy currency made in Krebs cycle
Reduction
Gain of electrons in a chemical reaction.
Oxidation (when molecule is oxidized)
Loss of electrons in a chemical reaction, releasing 2 electrons & 2 hydrogen ions
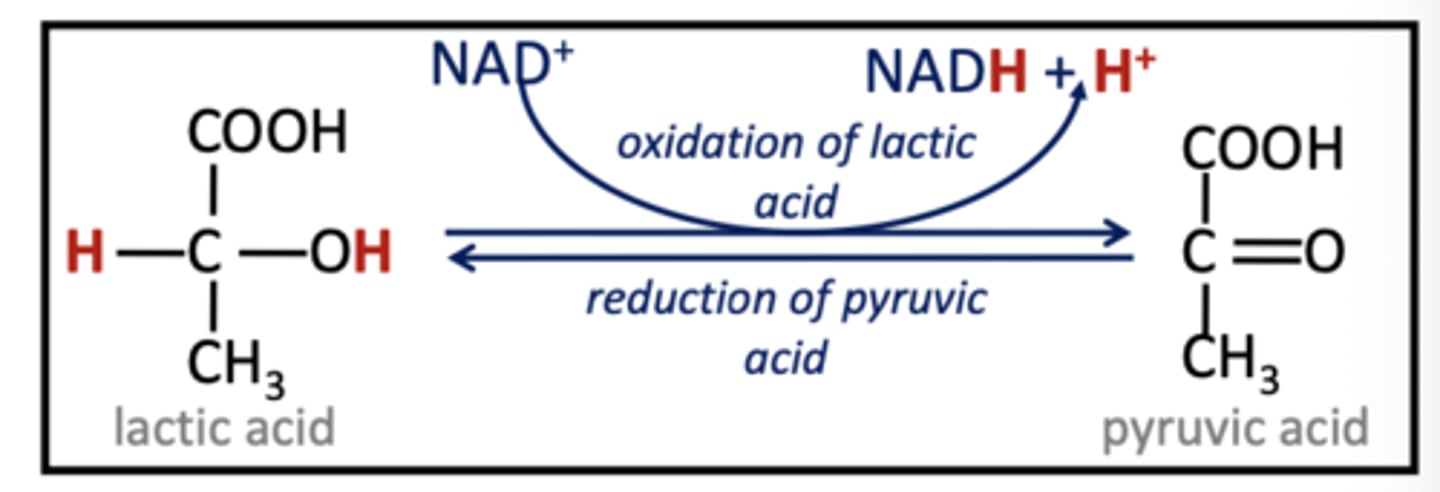
NAD+
Electron carrier derived from vitamin B3. (NAD+ picks up H+ & 2e-, remaining H+ becomes part of solvent)
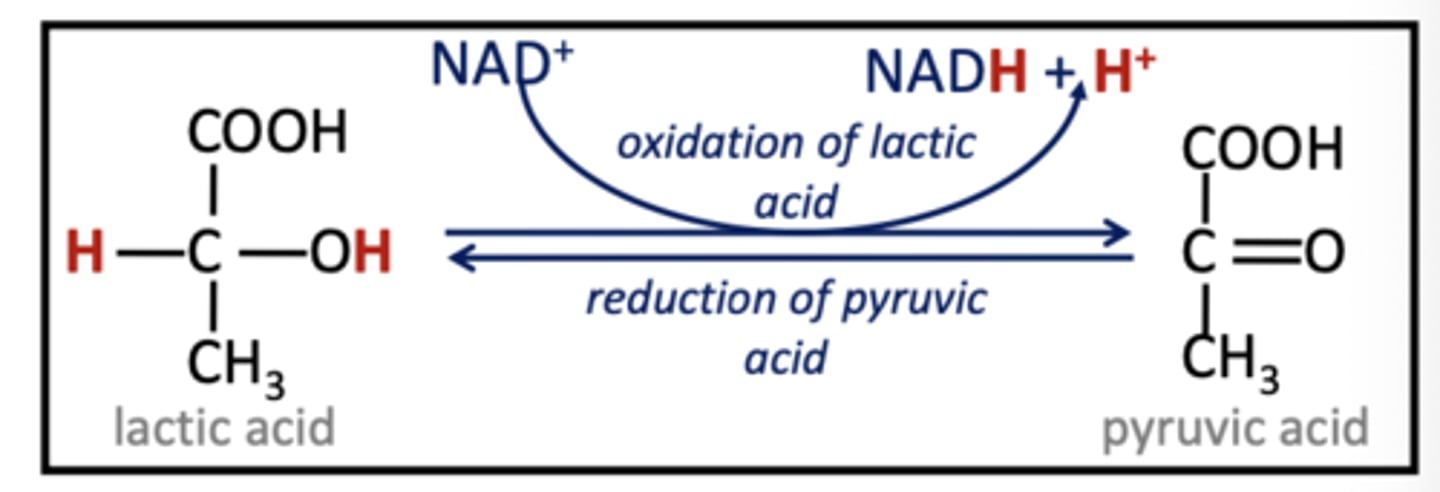
NADH (converted from?)
Reduced form of NAD+, carries electrons, coenzyme
FAD
Electron carrier derived from vitamin B2 (Picks up both electrons and hydrogens)
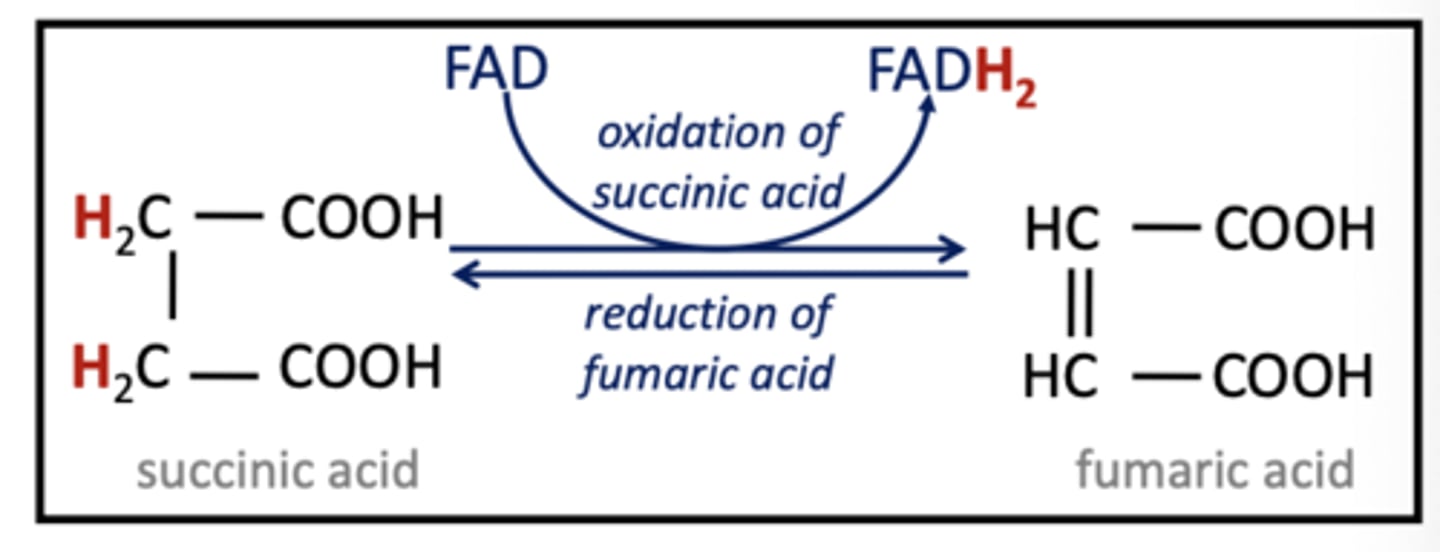
FADH2
Reduced from of FAD
cellular respiration (glucose metabolism) reactants & products
Glucose = nutrient molecule
Reactants:
• ADP, Pi = energy carriers
• NAD+, FAD = electron carriers
• O2 = required in the last step of cellular respiration
Products: CO2, ATP, NADH, FADH2, H2O
overall cellular respiration summary (chemical equation)
C6H12O6 + 6O2+ 32 ADP + 32 Pi =
6CO2 + 6H2O + 32 ATP
stages of glucose catabolism
1. glycolysis 2. pyruvate oxidation 3. Krebs cycle 4. electron transport chain reactions
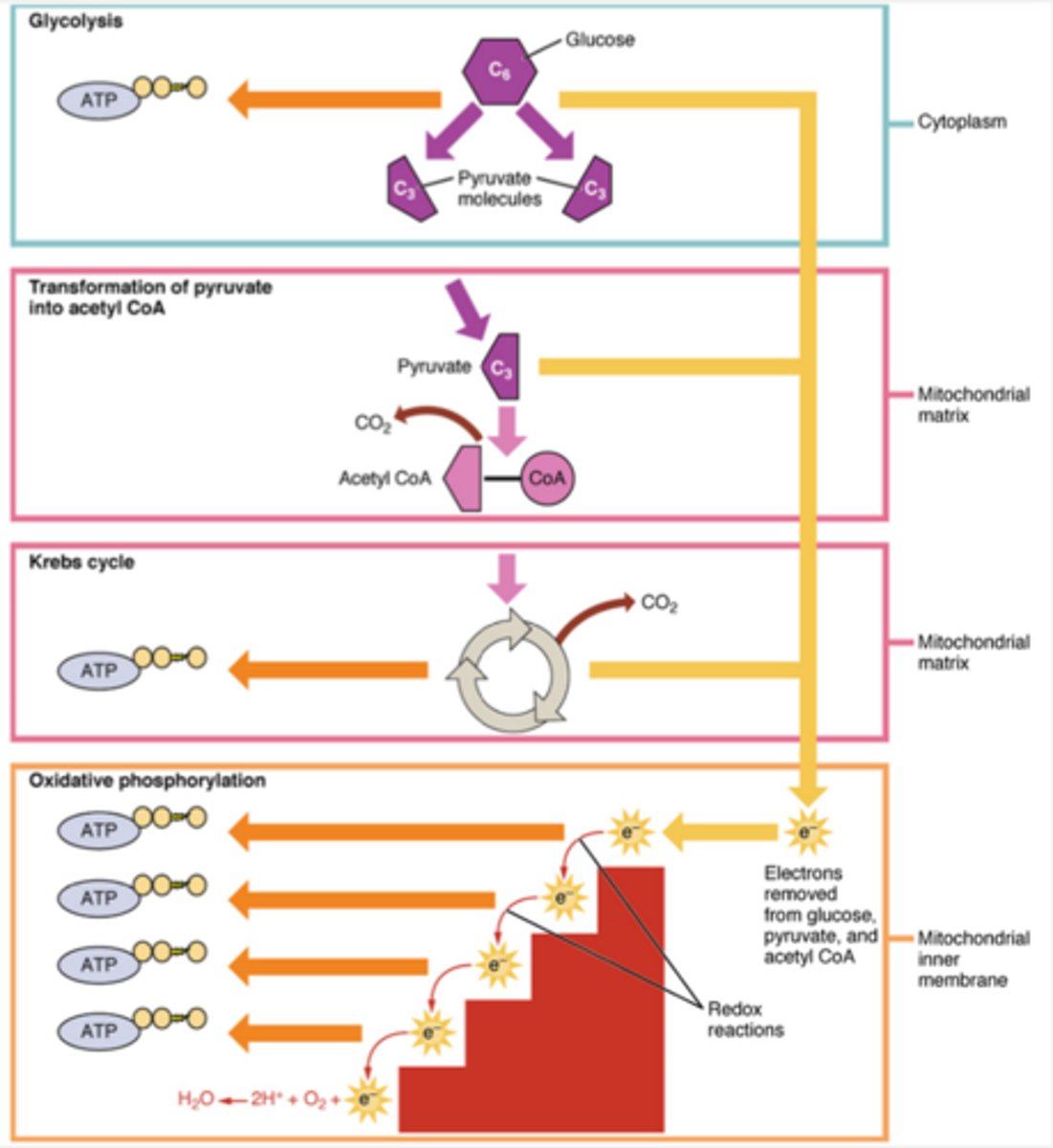
Glycolysis
First stage of glucose catabolism in cytoplasm.
Pyruvate Oxidation
Conversion of pyruvate to acetyl-CoA in mitochondria (mitochondrial matrix).
Kreb's Cycle
Series of reactions in mitochondria (mitochondrial matrix) for energy
Electron Transport Chain
Final stage of cellular respiration in mitochondria inner membrane
glucose uptake after eating
Food digested, glucose enters the bloodstream. Insulin secreted from pancreas that activates glucose transporters in cells, then glucose transported into cells for glucose uptake.
Stage 1 glycolysis
occurs in cytoplasm, multistep process converting glucose molecule (6 C) to 2 pyruvic acid (pyruvate) molecule (two 3 C)
Key events in glycolysis
1. Glucose is phosphorylated twice to trap
glucose into the cell
• Requires two ATP; energy investment step
2. Each reaction step is catalyzed by a
specific enzyme
3. The glucose molecule is oxidized to make
two pyruvic acid molecules
4. In total, four ATP and two NADH
molecules are produced in the end
5. Net total of two ATP and two NADH
molecules
glycolysis energy net total
1 glucose in. Get 2 pyruvic acid, 2 ATP, 2 NADH + 2 H+
Stage 2: Pyruvate oxidation
after glycolysis pyruvic acid transported to the mitochondrial matrix & oxidized further.
Key events in pyruvic acid
each pyruvic molecule is oxidized to make acetyl-CoA. During this 1 CO2 & 1 NADH is made.
Acetyl-CoA
an acetate molecule bound to coenzyme A (from pantothenic acid)
Energy net total from pyruvate oxidation
2 pyruvic acid in. Get 2 acetyl-CoA, 2 CO2, 2 NADH + 2 H+
stage 3: Kreb cycle
circular pathway that oxidizes nutrients to make energy. Each step is catalyzed by a separate enzyme. intermediates of the cycle can also be taken out & make the molecules. Occurs in mitochondrial matrix.
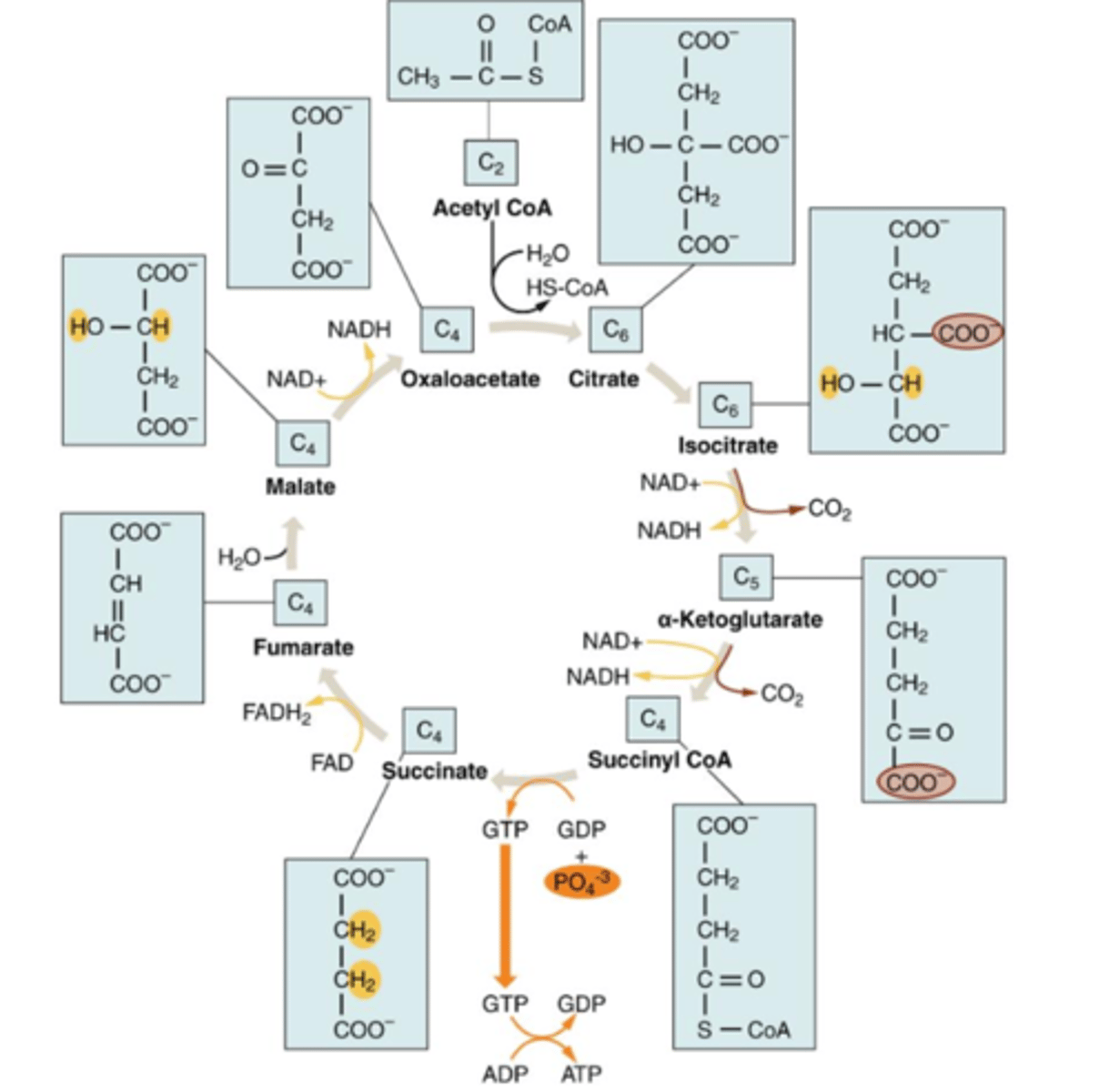
Key events in the kreb cycle
1 acetyl-CoA molecule enters the cycle by combining with a molecule called oxaloacetate, citrate formed, cycle continue & oxaloacetate regenerated. Ultimately acetyl-CoA molecule is converted to 2 CO2 molecules
other products of kreb cycle
3 NADH molecules, 1 FADH2, 1 ATP
Energy net total form Krebs cycle
2 acetyl-CoA in, get 2 CO2, 2 ATP, 6 NADH + 6 H+, 2 FADH2
other names for kreb cycle
citric acid cycle/tricarboxylic acid (TCA)
Stage 4: Electron transport chain
how oxidative phosphorylation occurs (energy in electrons passed onto ATP). Located on inner mitochondrial membrane.
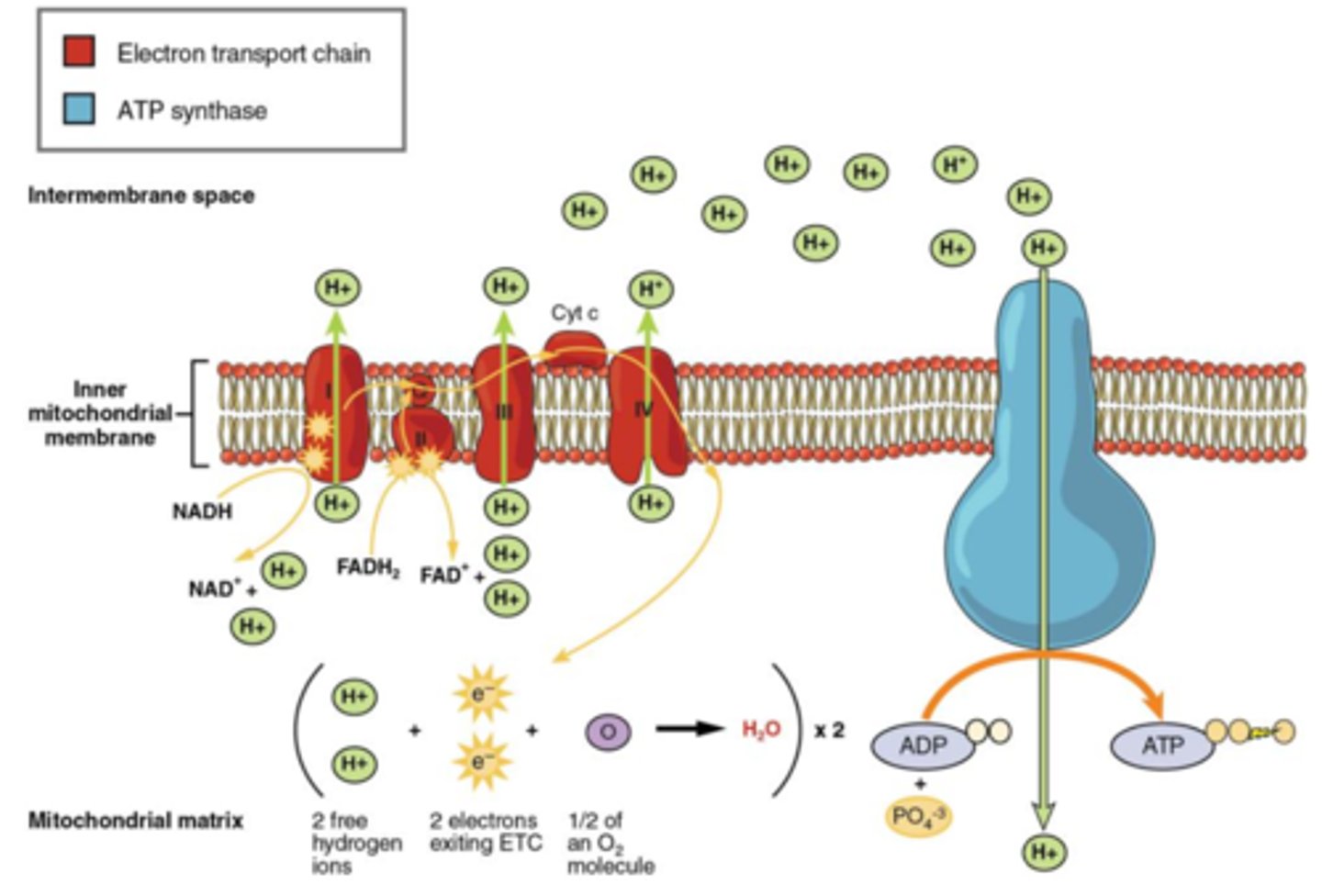
Electron Transport Chain (ETC) events
1. NADH releases its electrons at Complex I of the ETC. FADH2 releases its electrons at Complex II. Complexes I, III, and IV of the ETC are hydrogen ion pumps (proton pumps).
2. The electrons move from "high energy state" to "low energy state". Providing energy for Complex I to pump hydrogen ions against its concentration gradient from mitochondrial matrix into intermembrane space. The electrons get shuttled by protein Q to Complex III and then to
Complex IV by cytochrome C protein. Here the same thing happens at these
complexes where the energy released from the movement of these electrons
causes Complex III and IV to pump hydrogen ions into intermembrane space. 3. At the end of the ETC, the electrons combine with oxygen (final electron
acceptor) to form water. 4. The hydrogen ions in the intermembrane space then move down their
concentration gradient back through an enzyme called ATP synthase
(facilitated diffusion) and this movement of hydrogen ions causes ATP
synthase to produce ATP. Chemiosmosis
Oxidative Phosphorylation
Process of ATP production using electron transport.
Chemiosmosis
Process of ATP production using hydrogen ion gradient.
NADH Yield
NADH passes its electrons to the beginning of the electron transport chain (specifically to Complex I). Produces 2.5 ATP in the electron transport chain.
FADH2 Yield
FADH2 passes its electrons later down the chain (Complex II). Produces 1.5 ATP in the electron transport chain. Means less energy is released as electrons are passed along the protein chain.
Total ATP from Glucose & why usually fewer?
Theoretical yield is 32 ATP per glucose molecule. Some cells, the transport of NADH from cytosol to mitochondrial matrix leads to the loss of 2 ATP. Few ATP lost to instant energy needs & slight ineffeciencies in ATP synthesis process.
Why's anaerobic respiration needed?
Energy production without oxygen, occurs during heavy exercise (can't breathe oxygen fast enough for body to make ATP). The system would accumulate electrons, and with no final electron acceptor, the ETC would shut down, then too much NADH & FADH2 ( not more NAD+ & FAD).
Lactic Acid Fermentation
Conversion of pyruvate to lactate under anaerobic conditions.
Events of anaerobic respiration
Lactic acid fermentation (pyruvate to lactate), regenerates NAD+, allowing glycolysis to continue. However, pyruvate oxidation & Krebs cycle can't. Only 2 ATP produced from one glucose molecule. Once O2 is available lactate can be converted back to pyruvate.
Absorptive State
Period shortly after eating, nutrients absorbed & glucose used for energy
Postabsorptive State
Fasting state, body uses stored energy.
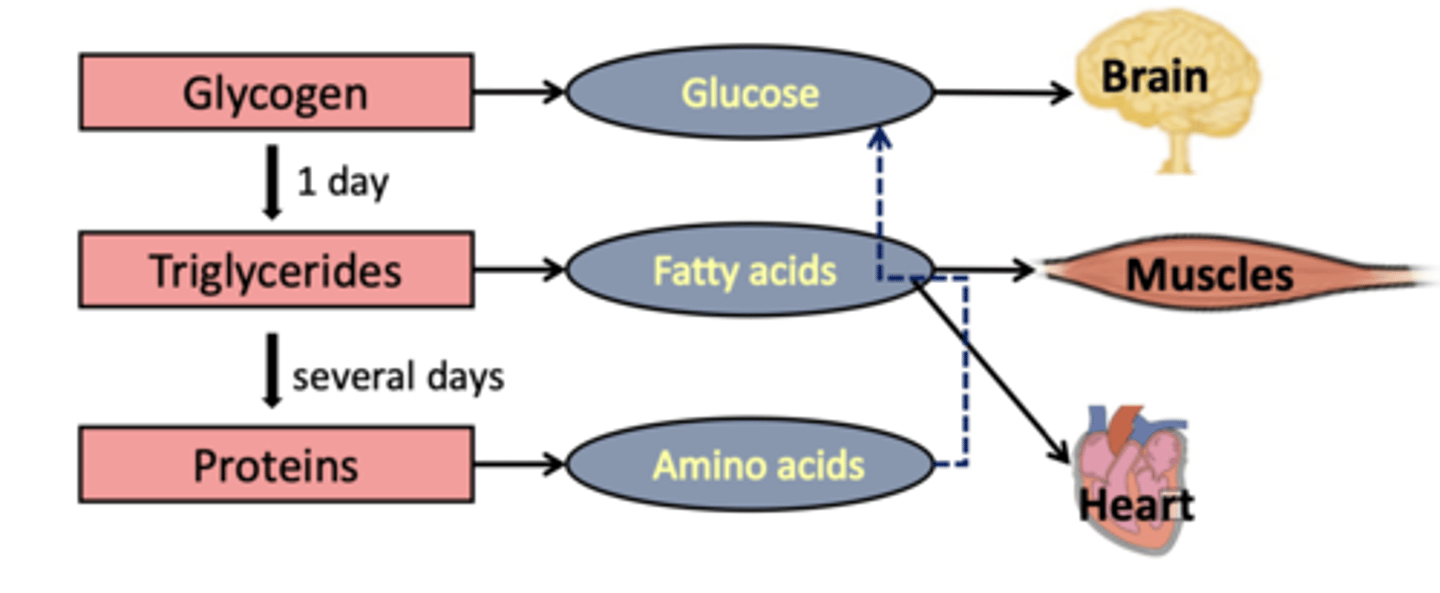
How excess nutrients are stored
Glucose → glycogen, Excess glucose → triglycerides, Lipids → triglycerides, Proteins → used for protein synthesis
1. Carbohydrate metabolism (excess/low levels)
first and usual source for energy source for most tissues. Blood glucose & glycogen sufficient for one day. When glucose is in excess: Glycolysis, Glycogenesis. When glucose is at low levels: Gluconeogenesis (liver only), Glycogenolysis
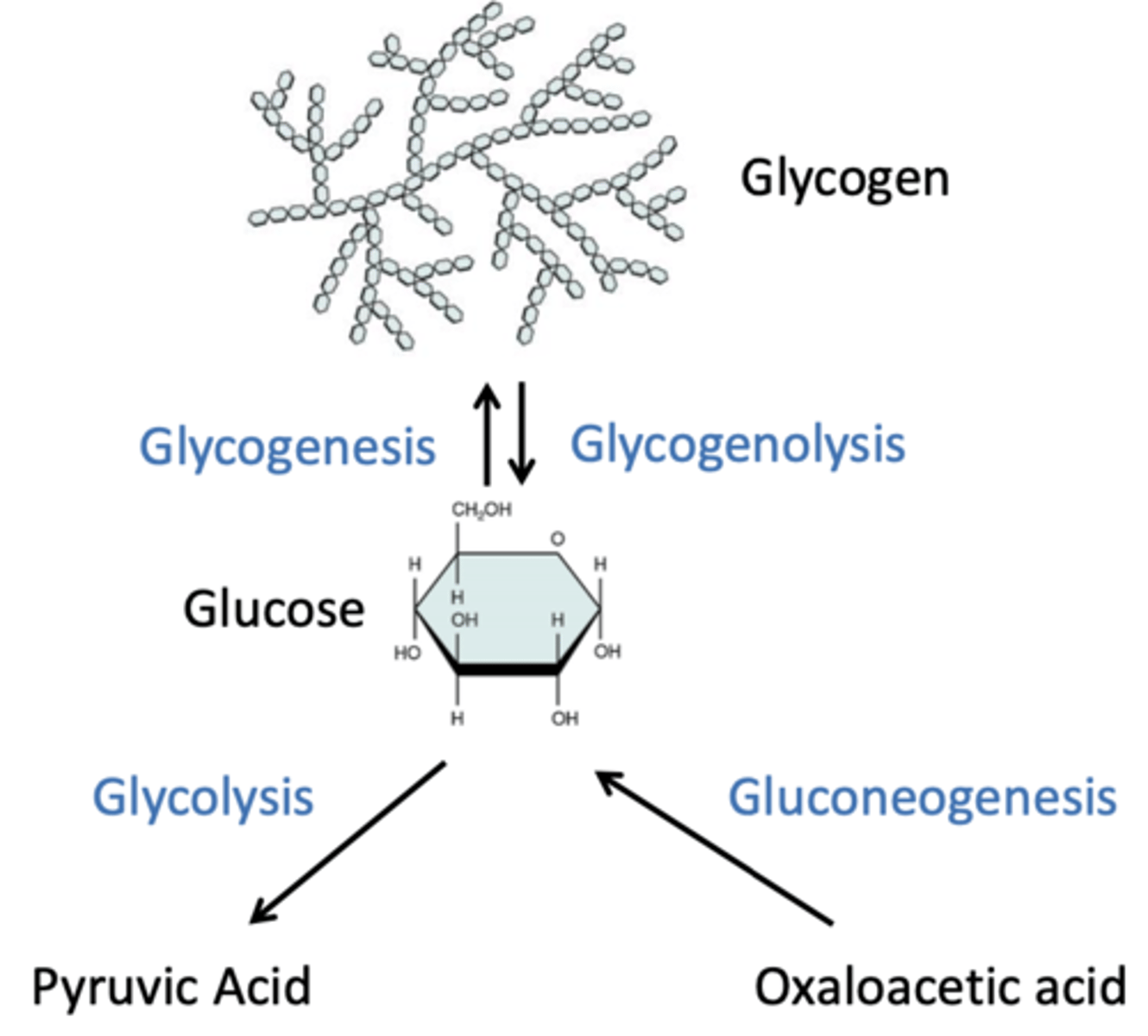
2. Lipid metabolism
triglycerides used when glucose levels fall, Except for the brain, which cannot use fatty acids. Some organs preferentially use fatty acids (liver, cardiac muscle, resting skeletal muscle)
Energy sources for the brain (how the brain survives)
It relies on glucose. When glucose runs low, the brain gets priority. The liver makes glucose for the brain from other molecules (gluconeogenesis). The liver also makes molecules called ketone bodies (ketogenesis), which can provide some energy for the brain
Ketone bodies
Energy sources produced from excess acetyl-CoA. they're acidic
Ketogenesis
Liver process producing ketone bodies from acetyl-CoA.
Processed involved in lipid metabolism (excess/levels fall)
When nutrients are in excess: An excess of glucose, amino acids, or lipids can be stored as triglycerides. When glucose levels fall: Triglycerides are broken down to glycerol and individual fatty acids → lipolysis. Glycerol can be used in gluconeogenesis Fatty acids are broken down to acetyl-CoA molecules → beta oxidation
Lipolysis
Breakdown of triglycerides into glycerol and fatty acids.
Beta-oxidation
Fatty acids broken down to acetyl-CoA molecules.
Extra processes in the liver (downsides of gluconeogensis/fasting)
Under normal circumstances, acetyl-CoA produced from fatty acids enter the Krebs cycle where ATP, NADH, FADH2 is then made. But the liver also performs gluconeogenesis, involves the use of oxaloacetate. Under extended fasting, all of the oxaloacetate from the Krebs cycle is shunted towards gluconeogenesis. With a key component missing, the acetyl-CoA produced from fatty acids have nowhere to go
Ketone bodies (excess of acetyl CoA)
Excess of acetyl-CoA leads to the production of ketone bodies (ketogenesis) by the liver: Acetoacetate, 3-hydroxybutyrate, Acetone. Ketone bodies then leave the liver and transported to other tissues, then converted back to actyel-CoA & used for energy (except acetone).
Acetoacetate
First ketone body produced during ketogenesis.
3-hydroxybutyrate
Second ketone body, used for energy in tissues.
Acetone
Third ketone body, exhaled, not used for energy.
Importance of Ketone bodies
to help minimize gluconeogensis and save protein catabolism.
Ketoacidosis
Acidic condition from excess ketone bodies in blood, can be deadly if extended.
Ketoacidosis can be a result of:
starvation, lack of carbohydrates in the diet, uncontrolled diabetes mellitus.
Protein catabolism
under extended fasting, glucose & fatty acids come low, proteins can be broken down for energy. They're convert to acetylene-CoA, & used for gluconeogenesis.
Fasting sequence
Order of nutrient use: glycogen broken down to glucose, lipids broken down tot glycerol & fatty acids (oxidized for energy), eventually before lipid stores run out protein breakdown occurs.