Organic Chemistry: Hydrocarbon Chains, Bond Types, and Molecular Geometry
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
46 Terms
Methyl
1
Ethyl
2
Propyl
3
Butyl
4
Pentyl
5
Hexyl
6
Heptyl
7
Octyl
8
Nonyl
9
Decyl
10
A pi bond makes a
Double Bond
Sp3
Tetrahdedral
Sp2
Trigonal planar
Sp1
Linear
What is the angle of SP3?
109
What is the angle of SP2?
120
What is the angle of SP?
180
Formal Charge
VE - (B+D)
Covalent
Nonmetal
Ionic
Metal
Cis isomer
Same dimension
Trans
Different Dimension (Zigzag)
Increasing Acidity trend
MORE EN = More acidity
Bronsted Acid
Acids Donate Protons
Lewis Acid
Accepts Electrons
Higher PKA
Weaker Acid
The pKA that is favored is the _________
Weaker one
The higher PKA is
Basic
The Lowest Boiling Point has
More Branches
High Boiling Point
Large # of Carbons
-OH
High boiling point
Bases have
lone charges on EN atoms
Acetal:
-OR
Amine
-NH3
Aldehyde
C=O-H
Alkene
C=C
Alkyne
C triple bond
Alcohol
-OH
Dipole Dipole
Positive and negative attracted to eachother
Hydrogen bonding
H bonded to N, O or F
EN increases
Going up
Prefix of Carbon ring
Cyclo-
Prefix
-Iso
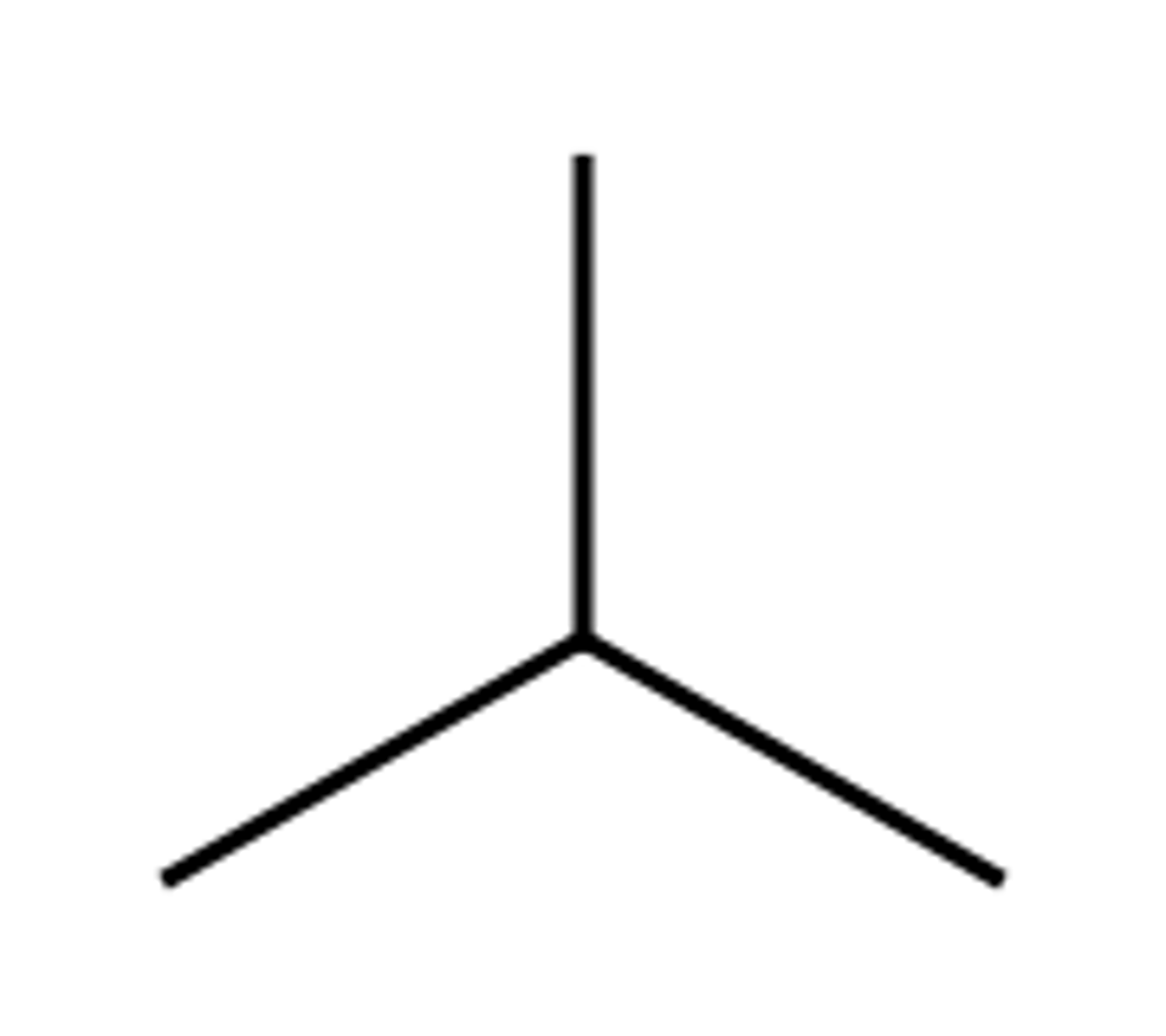
Prefix
-n
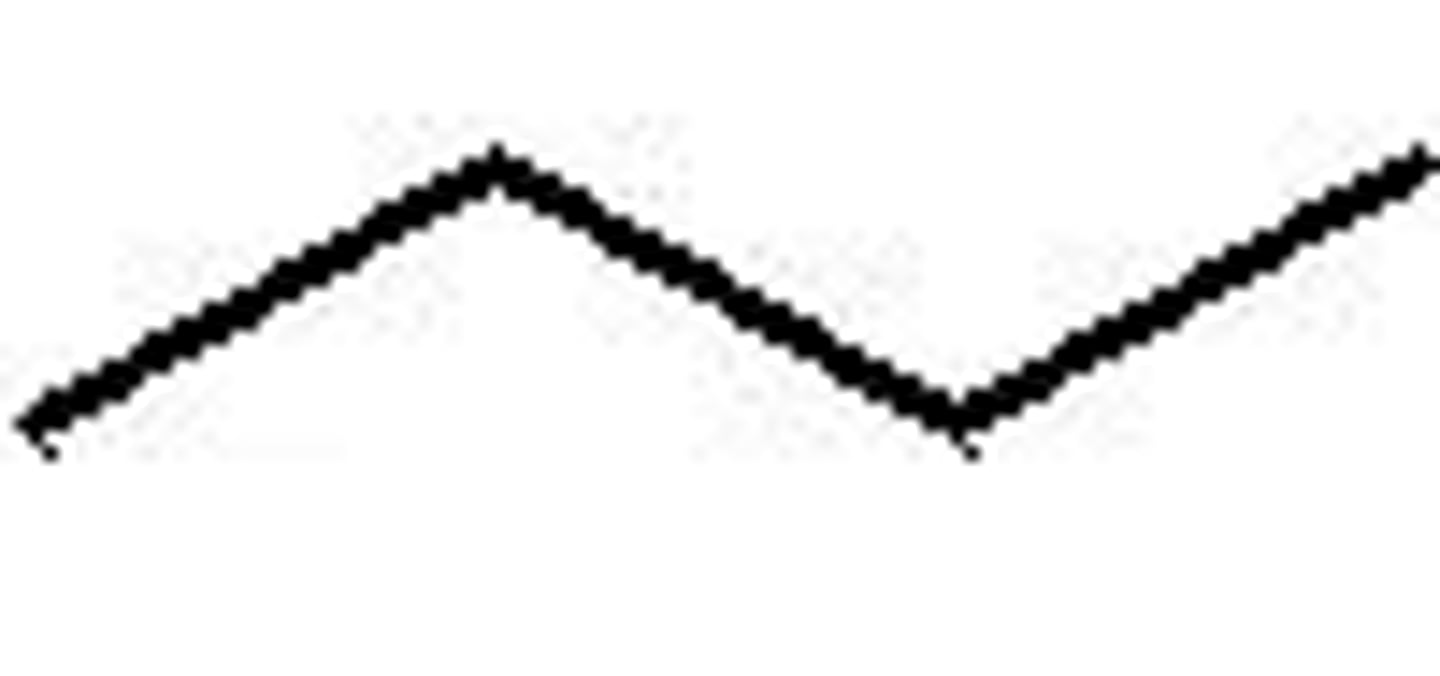
Prefix
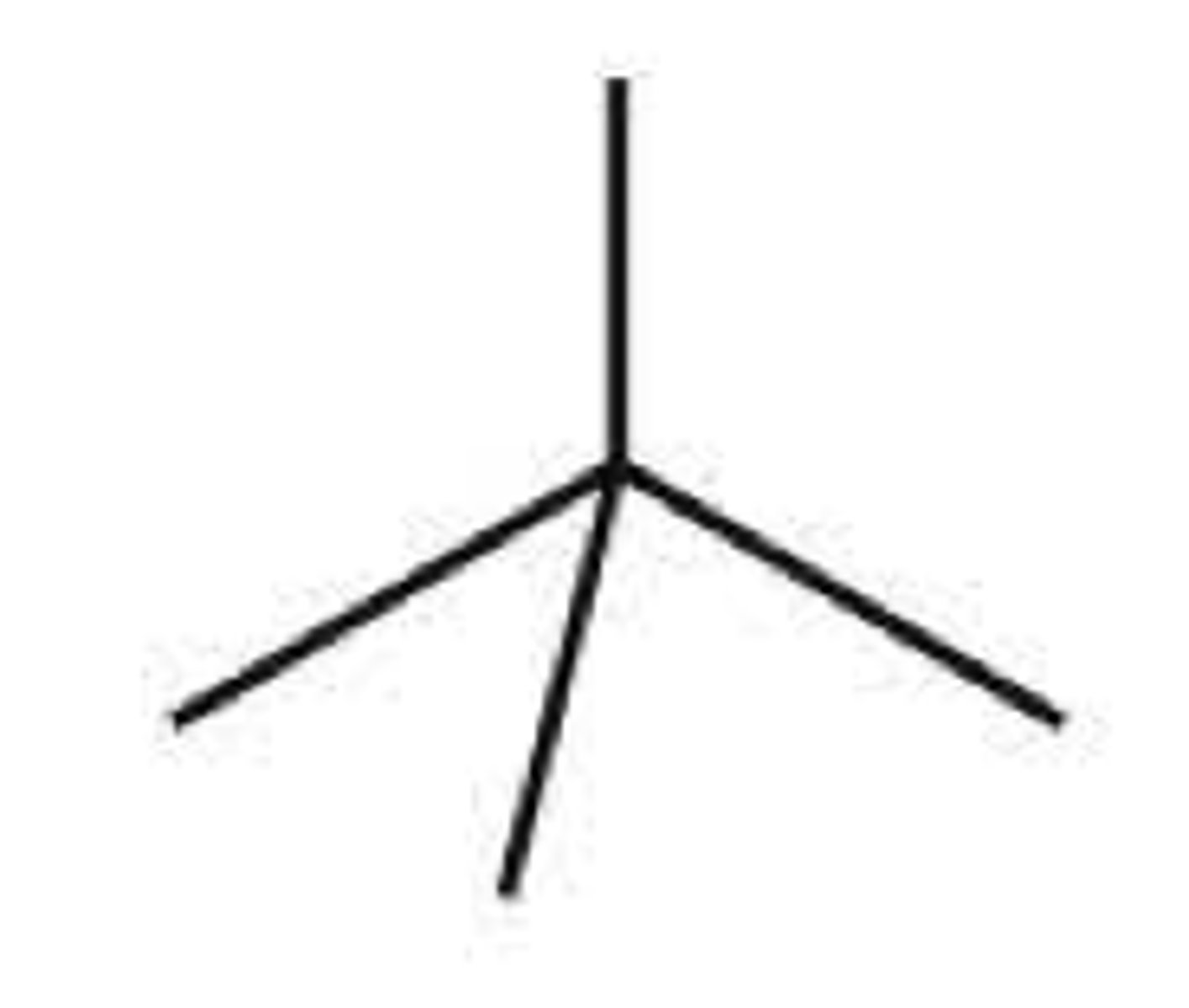
-neo
Axial (on a chair)
Straight Up or Down