S2.2.10: Chromatography
1/5
Earn XP
Description and Tags
i hope i dye
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
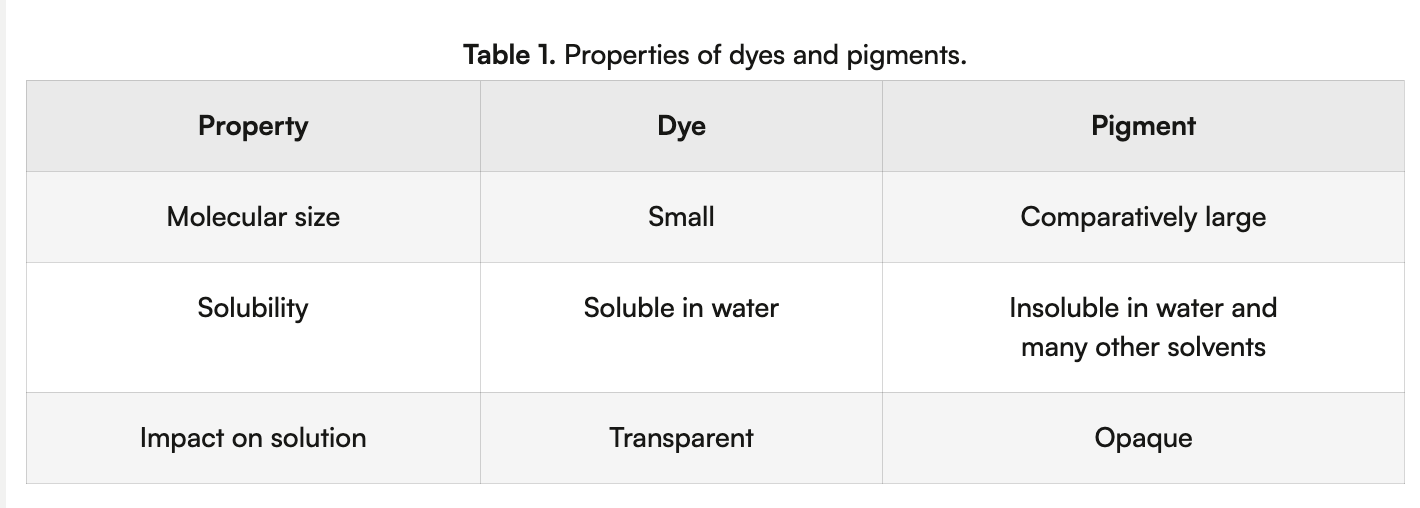
dye vs pigment
Dye molecules will typically dissolve in a polar solvent such as water and produce a transparent solution.
When mixed with a solvent the pigment molecules will remain suspended in the solution, producing an opaque solution. To dissolve pigments, often another chemical known as a ‘binder’ is added to the pigment.
chromatography
stationary phase and the mobile phase. The components of the mixture will either have an attraction for the mobile phase or stationary phase, based on the intermolecular forces present, which allows for effective separation.
how does chromatography work
mixture will travel up the chromatography paper as it is attracted by the water molecules trapped in the paper's fibres. exist in equilibrium between the hydrogen bonds it forms with the solvent (the mobile phase) and the intermolecular forces it forms with the paper (the stationary phase) (Each substance in the mixture is constantly “deciding” where to spend time:
Sticking to the paper (via H-bonds / intermolecular forces with the stationary phase).
Dissolving in the solvent (mobile phase)
This will determine how far up the paper it travels: the amount of time spent in each of the phases is a reflection of the strength of the intermolecular forces that are experienced in each phase. If a substance has strong intermolecular forces with the stationary phase, then it will remain adsorbed for much longer than if it has weak intermolecular forces.
retardation factor, retention factor, or RF value
distance each component in the mixture travelled relative to the distance the solvent travelled
Paper Chromatography
uses a piece of high-quality filter paper as its stationary phase. The paper's cellulose fibers are polar and naturally hold onto a thin film of water, the stationary phase. The separation of a mixture occurs via a partitioning mechanism. The different components of the mixture partition themselves between the stationary water and the moving mobile solvent. Polar components are more soluble in the stationary water layer, so they move more slowly and travel a shorter distance. Conversely, less polar components are more soluble in the mobile solvent and are carried further up the paper.
Thin Layer Chromatography (TLC)
rigid plate coated with a thin layer of a solid adsorbent, typically silica gel, as its stationary phase. The separation is based on an adsorption mechanism. As the mobile solvent moves up the plate, the components of the mixture are separated based on how strongly they adsorb to the polar surface of the silica gel. More polar compounds have a higher affinity for the polar stationary phase, causing them to move more slowly and stay closer to the origin. Less polar compounds have a weaker attraction to the stationary phase and are carried further up the plate by the mobile solvent.