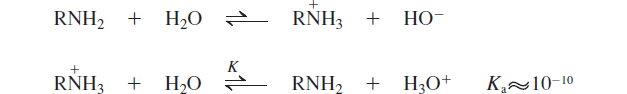Orgo 2 Exam 2
1/165
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
166 Terms
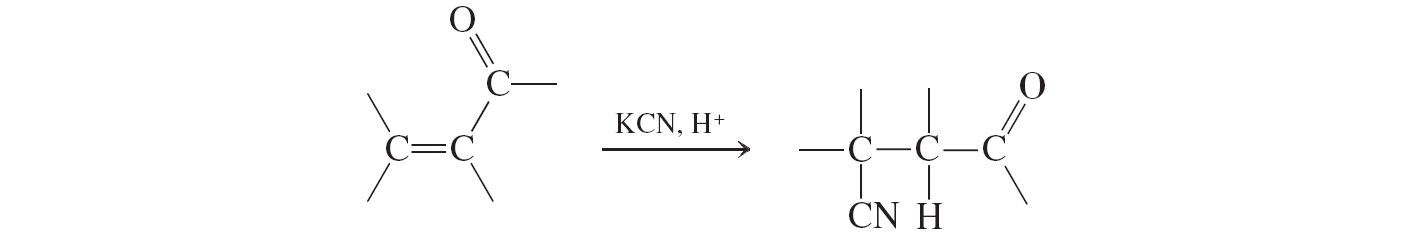
hydrogen-cyanide conjugate addition
1,2 addition reversible; 1,4 addition more stable
conjugate hydration (ether formation)
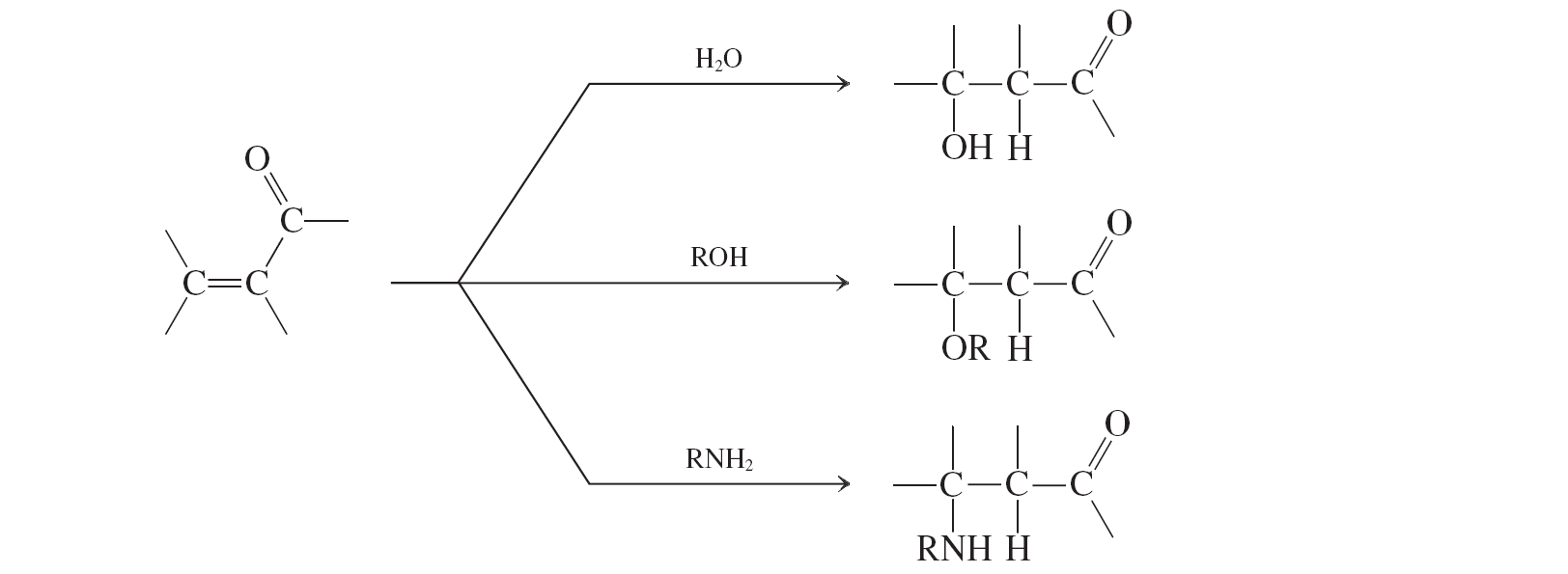
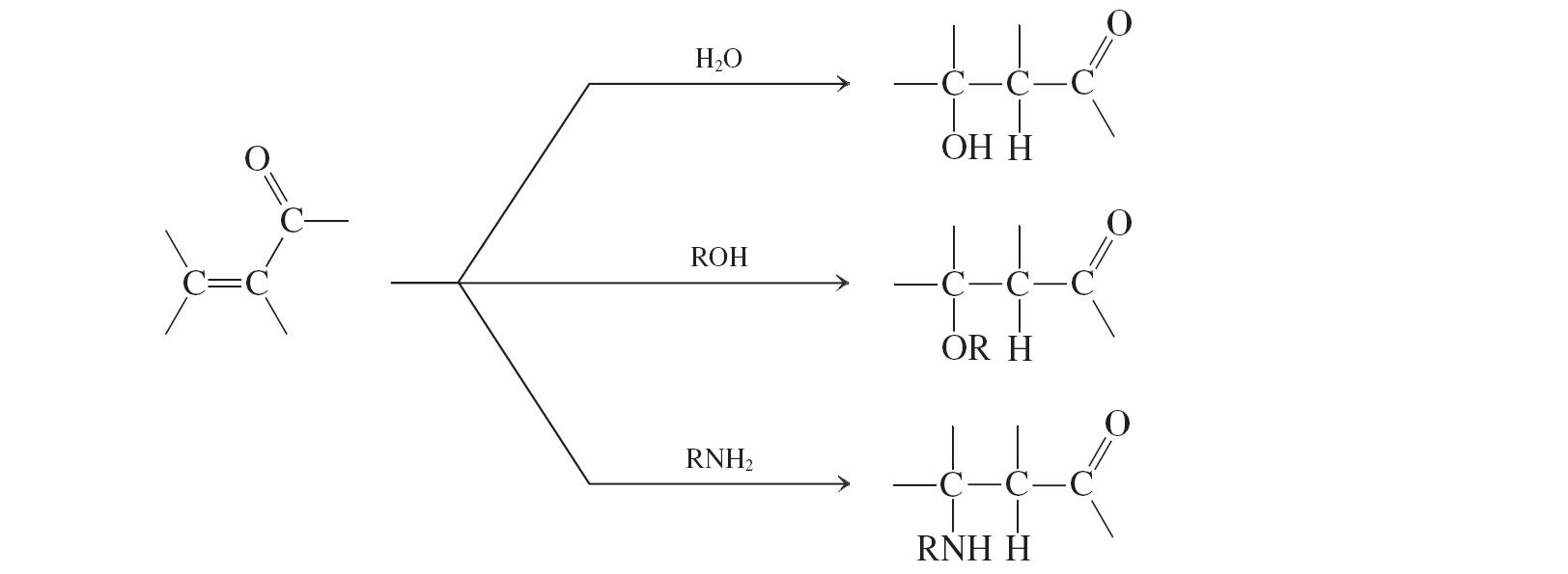
water, alcohols, and amines
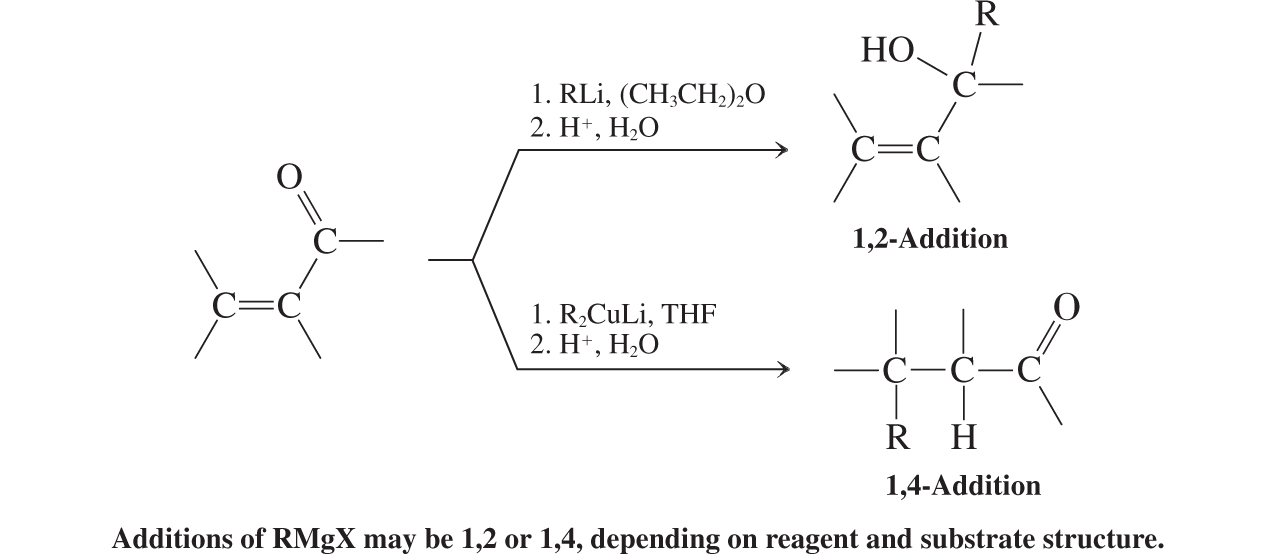
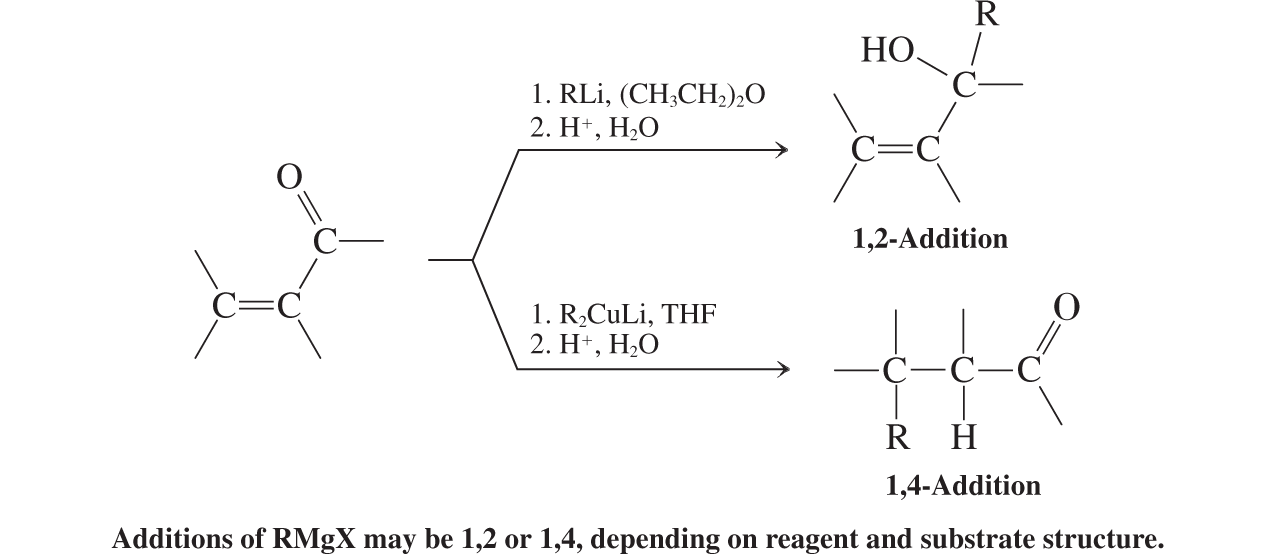
Organometallic Reagents
Intramolecular aldol condensation
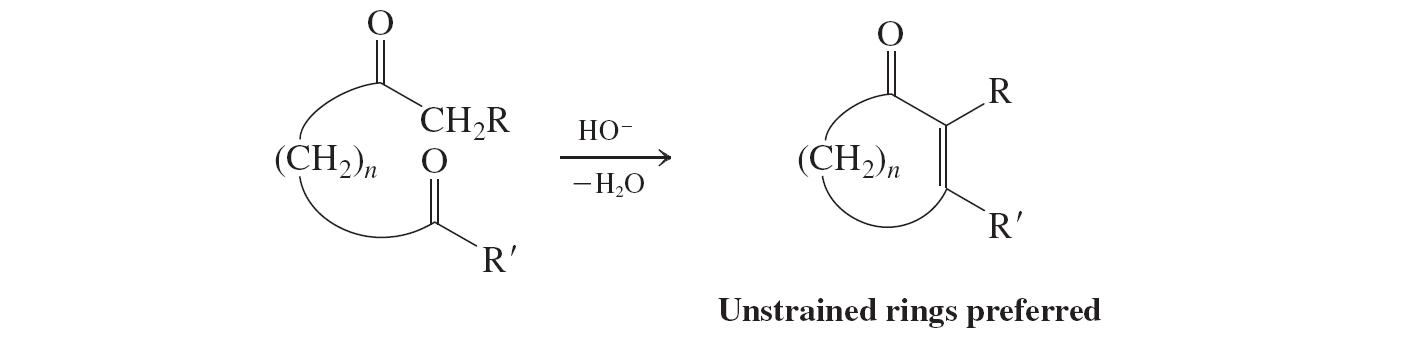
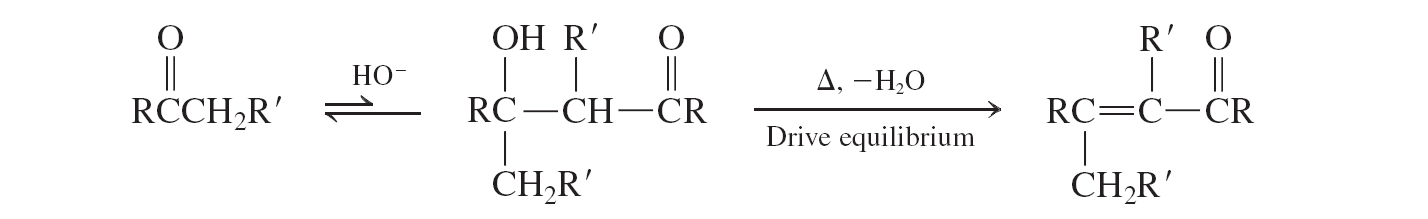
Ketons (aldol condensation)
Crossed aldol condensation (one aldehyde not enolizable)
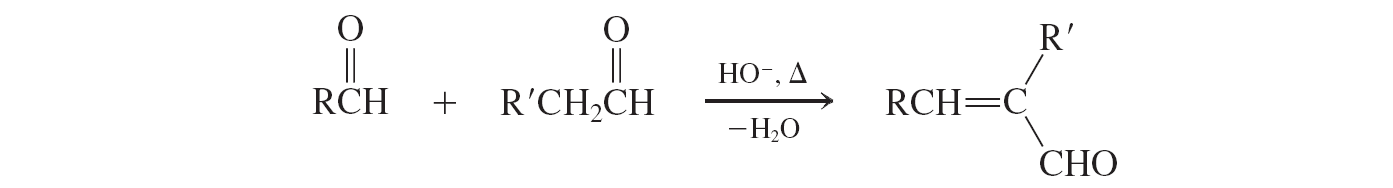
Aldol condensation
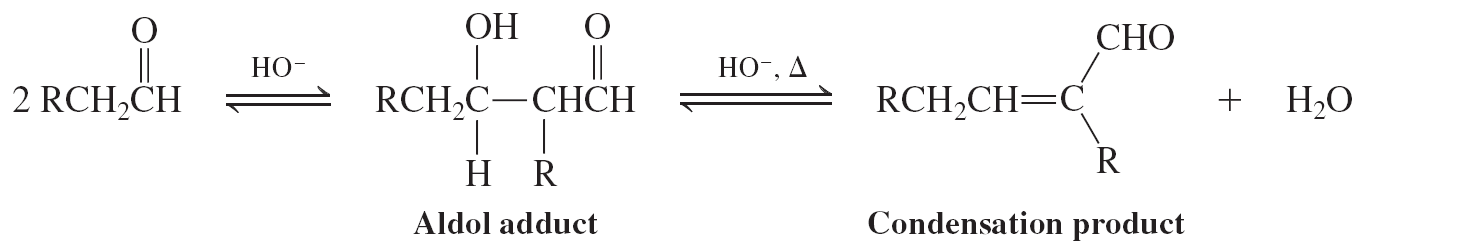
Hydrogenation (a,b unsat. aldehydes and ketones reactions)
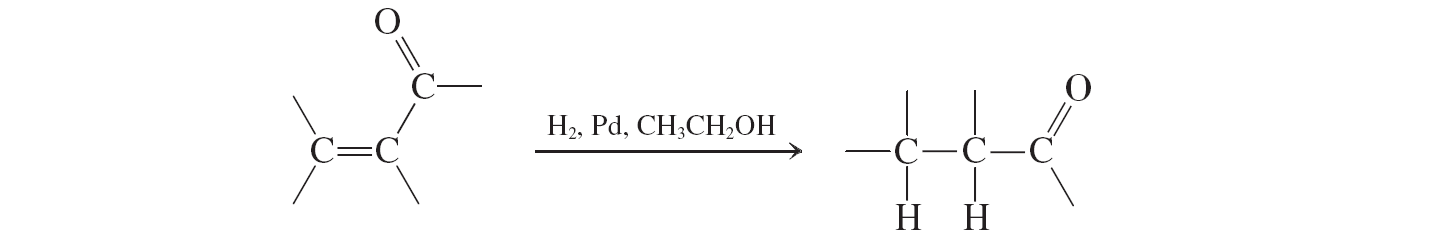
Addition of Halogen (a,b unsat. aldehydes and ketones reactions)
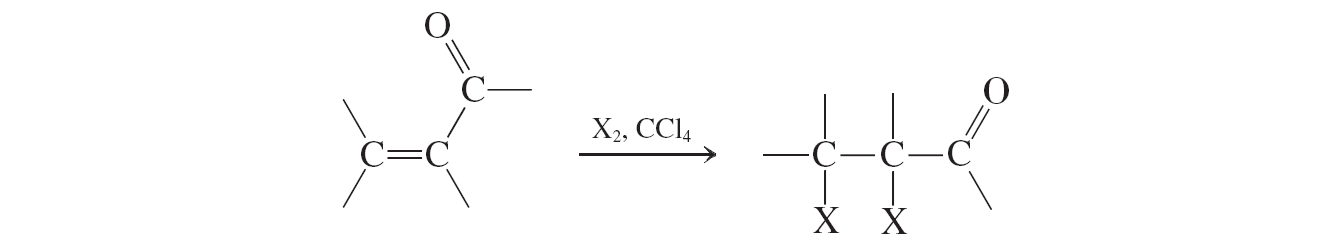
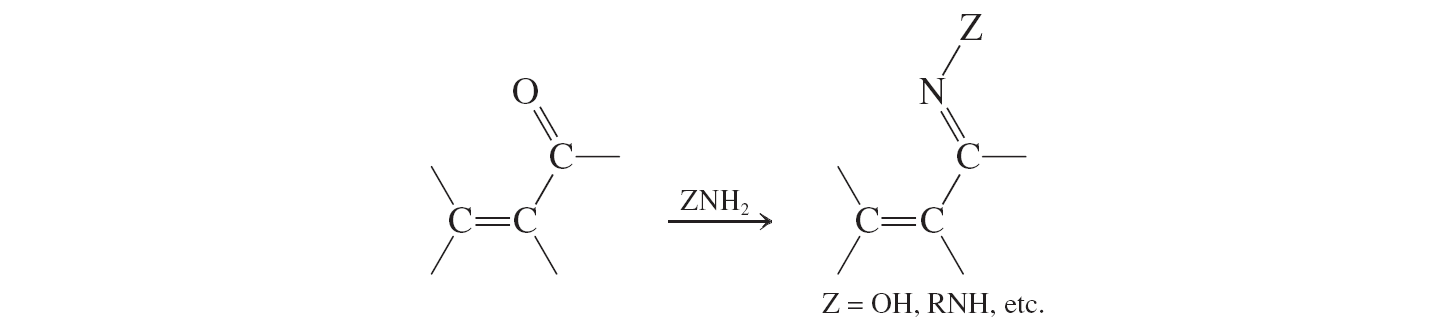
Condensations with Amine Derivatives (a,b unsat. aldehydes and ketones reactions)
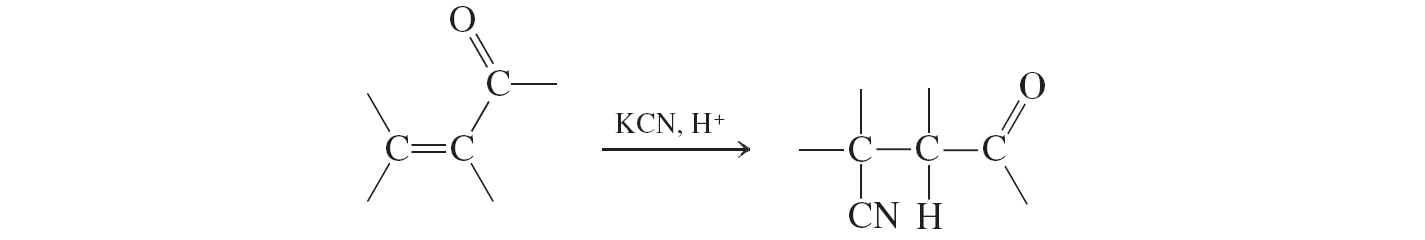
Hydrogen Cyanide Addition (conjugate additions to a,b unsat. aldehydes and ketones)
Water, alcohols, amines (conjugate additions to a,b unsat. aldehydes and ketones)
Organometallic Reagents (conjugate additions to a,b unsat. aldehydes and ketones)
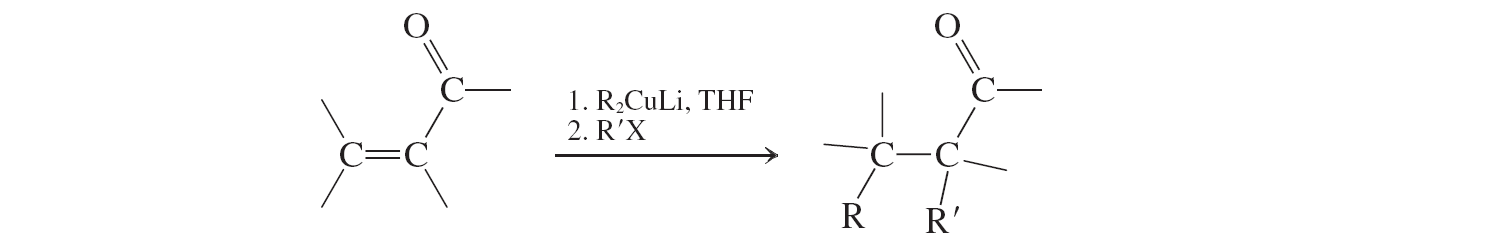
Cuprate additions, enolate alkylations (conjugate additions to a,b unsat. aldehydes and ketones)
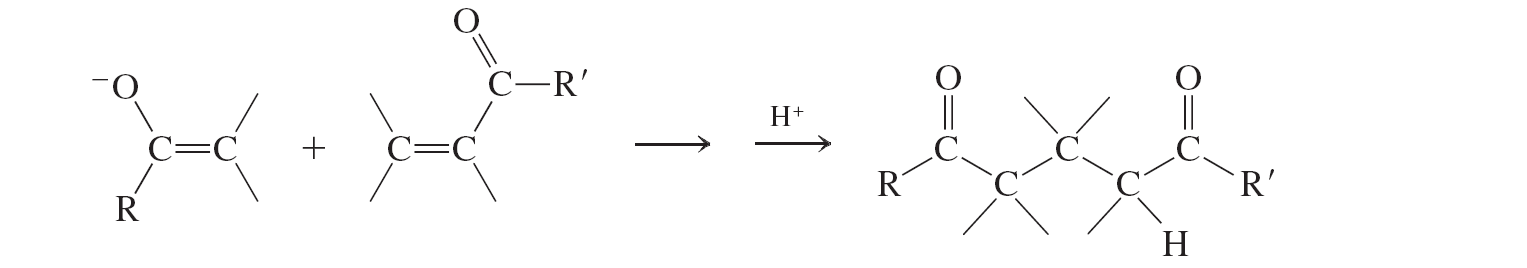
Michael Addition (conjugate additions to a,b unsat. aldehydes and ketones)
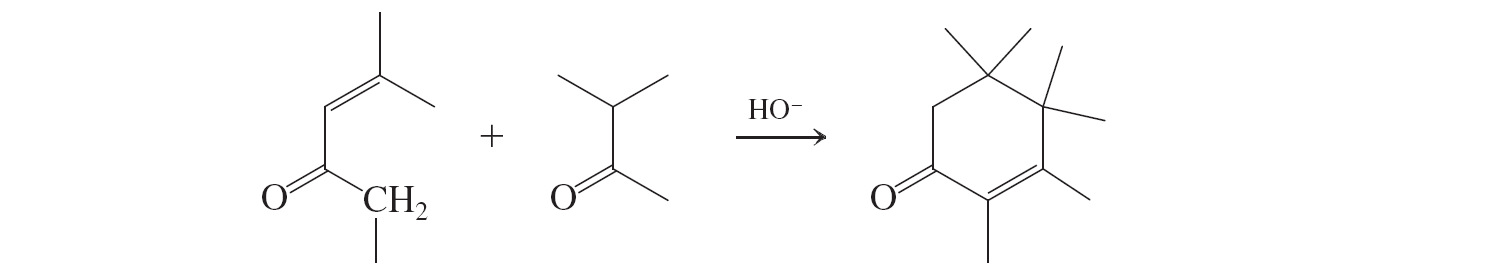
Robinson Annulation (conjugate additions to a,b unsat. aldehydes and ketones)
alpha hydrogens
acidic (due to e- withdrawing of fg and resulting enolate ion is resonance stabilized)
Unsymmetrical ketones generate ___ with LDA at low temps
less substituted kinetic enolate
Unsymmetrical ketones generate ___ with a weak base at high temps under equilibrating cond.
more substituted thermodynamic enolate
haloalkanes prefer
alpha-alkylation
protonation of oxygen leads to
enols
enamines
neutral analogs of enolates
a-halogenation maybe be
acid or base catalyzed
acid catalyzed a-halogenation
the enol is halogenated by attack at the double bond and the enolization is slowed down by the halogen substituent
base catalyzed a-halogenation
the enolate is attacked at the carbon and the enolate formation is accelerated by halogens introduced
enolates are…
electrophilic and reversibly attack the carbonyl carbon of an aldehyde or ketone in aldol condensation
attack the b-carbon of a a,b-unsat. carbonyl compound in michael addition
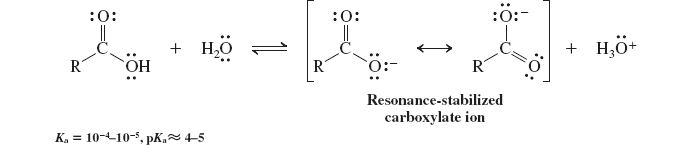
acidity of carboxylic acids
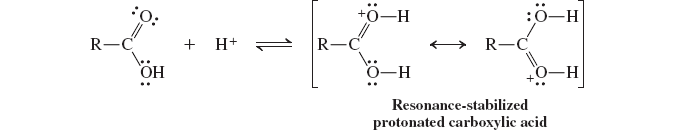
basicity of carboxylic acids
RCH2OH—?—→RCOOH (oxidization)
oxidizing agents: aqueous CrO3, KMnO4, HNO3
RCHO—?—→RCOOH (oxidization)
oxidizing agents: CrO3, KMnO4, Ag+, H2O2, HNO3
RMgX+CO2 —?→ RCOO- +MgX —?→ RCOOH (carbonation of organometallic reagents)
THF
H+,H2O
RLi+CO2 —?→ RCOO- +Li —?→ RCOOH (carbonation of organometallic reagents)
THF
H+,H2O
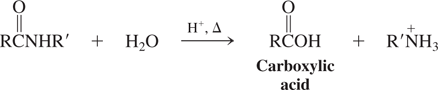
RCN—?→ RCOOH + NH3(acid catalyzed)/NH4+(base catalyzed) (hydrolysis of nitriles)
H20,heat,H+,H+(acid catalyzed)/OH-(base catalyzed)
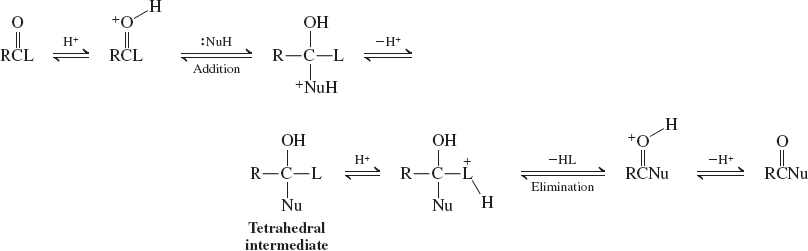
Nucleophilic attack at the carbonyl group (base catalyzed addition-elimination)(reactions of carbox. acid)
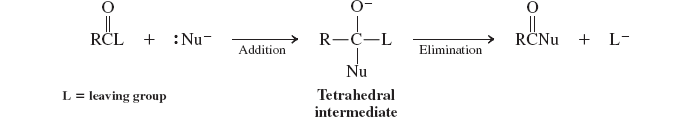
Nucleophilic attack at the carbonyl group (acid catalyzed addition-elimination)(reactions of carbox. acid)
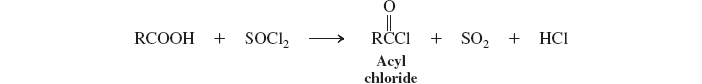
acyl halides (deriv. of carbox. acids) (SOCl2)
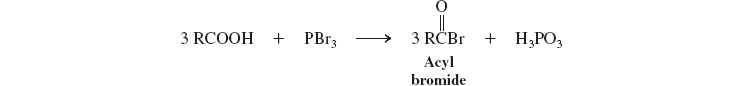
acyl halides (deriv. of carbox. acids) (PBr3)
Carboxylic Anhydrides
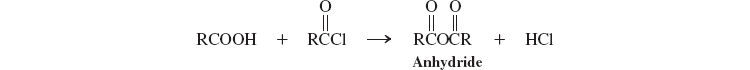
Cyclic anhydrides
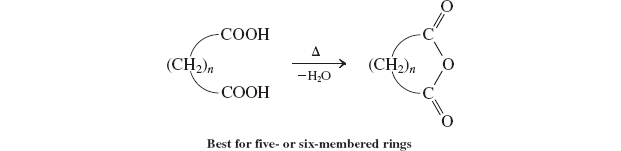
carboxylic amides
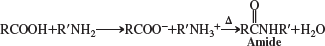
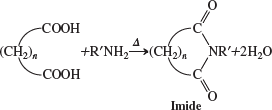
imides
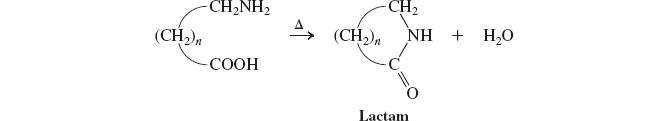
cyclic amides (lactams)
RCOOH —?→ RCH2OH (reduction with lithium aluminum hydride)
LiAlH4,(CH3CH2)2O
H+,H2O
Bromination: Hell-Volhard-Zelinsky Reaction
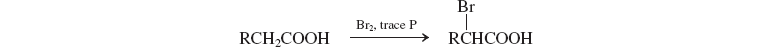
.
.
carboxylic acids
alkanoic acids
dicarboxylic acids
alkanedioic acids
cyclic and aromatic systems involving carboxylic acids
cycloalkanecarboxylic and benzoic acids
carboxy groups
trigonal planer
EXCEPT in dilute solutions and then it forms dimers by h-bonding
carbonyl group in carboxylic acids undergo nucleophilic displacement via ___
addition-elimination pathways
addition of nucleophile ______ that decomposes by elimination of the hydroxy group to _____
gives an unstable tetrahedral intermediate
give a carboxylic acid derivative
LiAlH4 is strong enough to add to the carbonyl group of ____ which allows the reduction of carboxylic acids to _____
carboxylate ions
primary alcohols
Order of Reactivity of Carboxylic Acid Derivatives
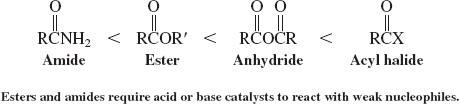
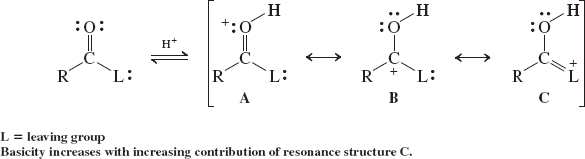
Basicity of the Carbonyl Oxygen
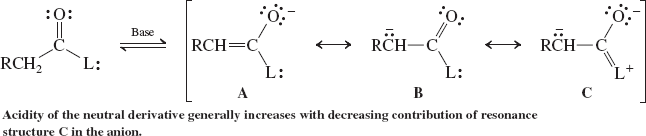
Enolate Formation
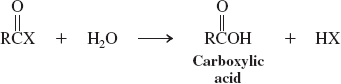
Water (reactions of acyl halides)
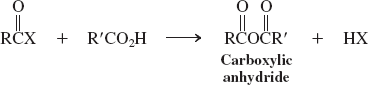
Carboxylic acids (reactions of acyl halides)
alcohols (reactions of acyl halides)
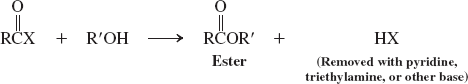
Amines (reactions of acyl halides)
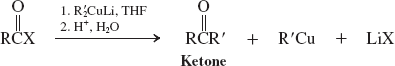
Cuprate Reagents (reactions of acyl halides)
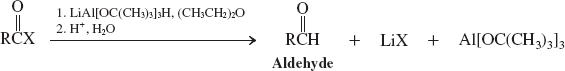
Hydrides (reactions of acyl halides)
Water (reactions of carboxylic acid anhydrides)
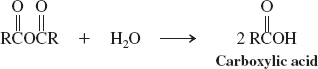
Alcohols (reactions of carboxylic acid anhydrides)

Amines (reactions of carboxylic acid anhydrides)

Water-acid catalyzed (Ester Hydrolysis) (Reactions of Esters)
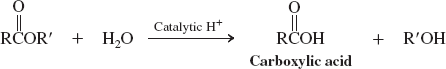
Water-base catalyzed (Ester Hydrolysis) (Reactions of Esters)
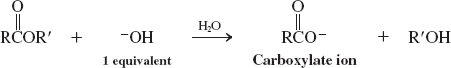
Alcohols (Transesterification) (Reactions of Esters)
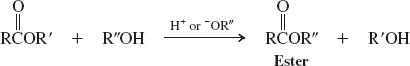
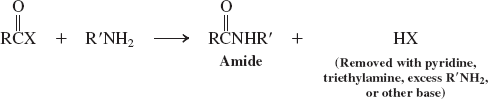
Amines (Reactions of Esters)
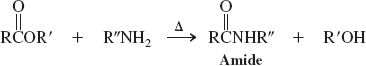
Organometallic Reagents (Reactions of Esters)
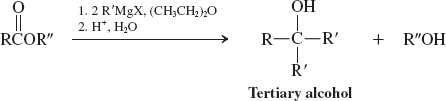
Methyl formate (Organometallic Reagents) (Reactions of Esters)
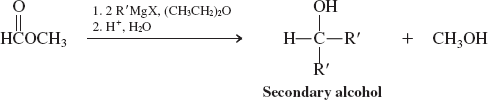
Hydrides-LiAlH4 (Reactions of Esters)
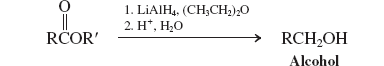
Hydrides-RAlH (Reactions of Esters)
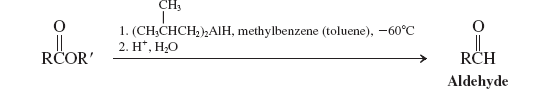
Enolates (Reactions of Esters)
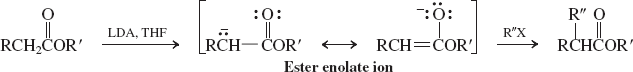
Water-acid catalyzed (Reactions of Amides)
Water-base catalyzed (Reactions of Amides)
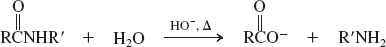
Hydrides-LiAlH4 (Reactions of Amides)
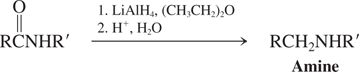
Hydrides-RAlH (Reactions of Amides)
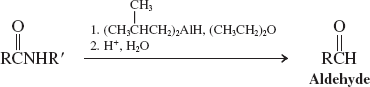
Enolates (Reactions of Amides)
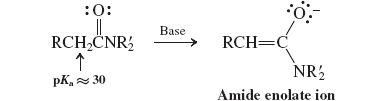
Amidates (Reactions of Amides)
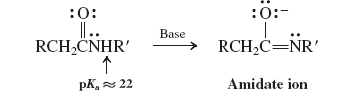
Hofmann Rearrangement (Reactions of Amides)
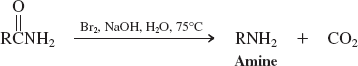
Water (Reactions of Nitriles)
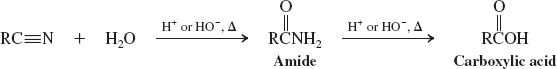
Organometallic Reagents (Reactions of Nitriles)
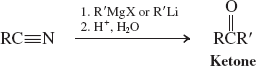
Hydrides-LiAlH4 (Reactions of Nitriles)
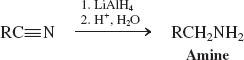
Hydrides-RAlH (Reactions of Nitriles)
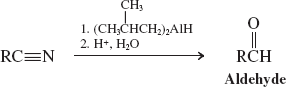
Catalytic Hydrogenation (Reactions of Nitriles)
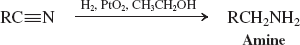
electrophilic reactivity of the carbonyl carbon in carboxylic acid derivatives is ____ by ____
weakened
good e- donating substituents
carboxylic acids derivatives
acyl halides
carboxylic acid derivatives
carboxylic anhydrides
ester derivative
alkyl alkanoates
amide of an alkyl carboxylic acid
alkanamides
alkyl halide with cyanide
alkanenitriles
carboxylic acid derivatives react with ___
water to hydrolyze the carboxylic acid
Carboxylic acid derivatives combine with ____ to give esters and with ____ to give amides
alcohols
amines
carboxylic acids form ketones with _____
grignard and other organometallic reagents
redcution of a carboxylic acid by hydride gives…
aldehydes, alcohols, or amines
transesterification
can be used to convert one ester into another
.
Acidity of Amines and Amide Formation
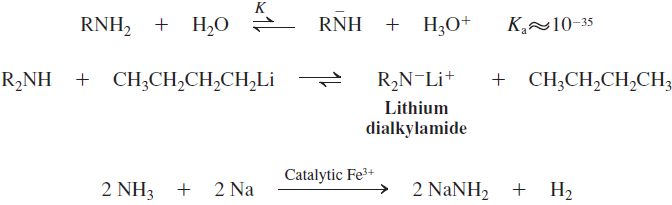
Basicity of Amines