no flowchart review
1/183
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
184 Terms
Ethical Issues from Epo Doping?
unfair advantage due to change in body’s abilities
It’s harmful to the athlete bc it inc BP to strain heart
Medecine is to be used for healing over personal gain
Parameters to consider when drug development to block a signal pathway
If you block at receptor, other downstream pathways may be disrupted that aren’t your target
Ex. cAMP is a general secondary messanger
Must consider:
Specificity to tissue
Specificity to pathway
Minimizing side-effects on normal cells
Beta-blockers
inhibit B-agrenergic receptors to reduce heart rate and BP
Used to treat hypertension, arrhythmias, anxiety
Has side-effects though
Taxol Function
Chemotherapy drug from pacific yew tree
Stabilizes microtubules to prevent depolymerization during mitosis
Arrests cell is G2/M phase and triggers apoptosis
Why do cancer drugs target microtubules rather than actin?
Cancer cells exhibit rapid division
Microtubules form the mitotic spindle essential in mitosis
Actin is more for shape and mobility
Targetting actin would harm all cells bc it plays improtant role there
Targetting microtubules is more to cancer bc it’s more sucesptible to it since it exhibits over proliferation
Why does cell adhesion matter in cell biology
cell-cell communciation to maintain tissue architecture
Immune Repsonse as immune cells must navigate and reach sites of infection
Pathogen strategies as they exploit adhesion to invade hosts
Therapeutic implications as understanding adhesion helps us design targeted therapies
UPEC
Causes UTIs by adhering to host epithelium
They use pili and adhesins to do this
Colonize / invade urethra and bladder
They repress the immune system there
Form biofilm and damage the epithelial
Can cause sepsis when it gets to major bloodstream
UPEC adhesion for UTI (Fim H)
Adhesion needed so they’re not swept away from the host
They use lectins to do so
These lectins are on the tip of the pili and can interact with mannose sugars on surface of urinary tract epithelial cells
Fim H is the lectin region that targets mannose
Fim H is necessary for this adhesion
UTI Treatment challenges
Treated by antibiotics
Challenges: Antibiotic resistance and perturbance of beneficial gut microbiome
Blocking UPEC adhesion to prevent UTI
Intriduction of competitive inhibitors
They bind to the lectin regions so they cannot adhere to cell surface
FimH binding domain can be used to design inhibitors with excellent potency
FimH: Large scale screens
Determines which inhibitors may work best to inhibit UPEC adhesions
Incubate UPEC with inhibitors
Pre-coat wells with mannose
After incubation, see which inhibitor doesn’t allow UPEC cell binding best
Cranberries in UTI Prevention
They have proanthocyanidins that are bound by FimH
UPEC adherence thus decreases with it
Limitations with Inhibiting FimH
Bacteria have manyyy pili making it difficult to block them all
They also express many adhesino molecules so FimH alone may not be enough
So, maybe we can develope an array of inhibitos as a therapy
Similarities and Differences with bact/WBC adhesion
Sim:
Similar movement to target tissue
Use adhesion to get places
Diff:
Integrin/Selectin for WBC while bact uses lectin to target mannos
Bact aggregate to damage while WBC go in to fight
Structure and Function of Kinesin Superfamily
Kinesin ½ bind cargo
Kinesin 5
4 ATP binding head chains
Allows sliding bc it walks along 2 at once
Kinesin 13
No motor activity
Helps in disassembly
MTOC
centrosome in regular cell
Located near nucleas and nucleates MTs
Becomes spindle poles during mitosis
Spindle Assembly
Each centrosome has a pair of centrioles
MTs nucleate in PCM which have y-TuRC where they polymerize
Centrosome duplication
occurs at G1/S when chromosomes are duplicating
G1/S phase CDKs initiate this duplication
In G2, daughter centriole growth is complete
Centrosome Splitting
M phase CDK activates this
Both MTOC nucleate and polymerize MTs
The two centrioles are pushed to opposite sides of nucleus and are now spindle poles
This happens during prophase before NEB
Mediated by Kinesin 5
Walks antiparallel (+) on two MTs allowing them to slide past
MT Types
Astral: Project and link to cell cortex
Kinetochore: Connect to chromosomes
Polar: Project toward cell center and overlap to help push spindle poles apart
How do MTs seperate sis chromatids during mitosis
Monopolar attachment of one sister chromatid
Bipolar attachment by 2 MTs
This tension aligns them at centre
MTs interact with the kinetochore protein complex of each sister chromatid
All chromosomes must achieve bipolar attachment to enter anaphase (SAC)
Anaphase A
Kinetochore microtubule shortening
Chromosomes are pulled poleward
Shortening of kinetochore MT pull them towards the spindle pole to seperate sister chromatids
Anaphase B
Seperation of spindle poles
Sliding force egenrated b/w polar MTs to push them apart
This acts on the poles to move them apart
Also MTs grow at the (+) to keep kinesin 5 to have the overlap needed to generate this sliding force
Cancer
Loss of normal control of cell proliferation
Large virable nuclei
Variation in size/shape
loss of normal specialized features
Large # of dividing cells
It’s mamed for which cell type it begins in
how are tumors different from normal cells
Changes in genomes (many types of mutations)
Tumours have different cell types that interact with their env to obtain max growth advanatge
Metastatic tumours have migrstory properties
causes fo cancer
Many small changes allowing the cell to be best-suited for uncontrolled growth
Acquired mutations are most common, from risk factors
Tobacco
UV
Toxins
Age
Germline mutations
Mutations in sperm/egg
Passes from parent to child so inherited cancer
Mutations for cancer
cancer is from many mutations over a lifetime
1. tumor suppressor genes
Moniter cell division
repairs mismatched DNA
Ex. BRCA1/2
Ex. p53 or TP53
Most p53 gene mutations are acquired
2. Oncogenes
Turn healthy cells cancerous when mutated
Ex. HER2
Ex. RAS
3. DNA repair genes
Fix mistakes when DNA is copied
Mutations here is a proble
Ex. BRCA1/2
Deregulation of cell cycle in Cancer maintenance
Overexpression of proto-oncogenes
LOF mutations in tumor suppressors
LOF in p53
Mutations in genome-maintenance genes
Cancer treatments
Radiation therapy: High dose of rad to kill and stop spread
Chemotherapy: Chemical killer to weaken cells
Surgery: Removing tumor
Immunotherapy
Cancer drug development
it’s becoming more prevelant in younger people too so new promising therapies may not be so effiicent
Drug Resistance
Cell pumps out toxins so it’ll pump out this toxin and over-express the mechanism for it for next time
Repopulation (they’ll come back after dying)
Drug distribution (one exposure to it can cause it to mutate to not be vulnerable)
p53
tumour suppressor and TF regulating cell devision to prevent tumors
Stops cells with damaged DNA from dividing and signals apoptosis
Or it arrests the cells if not too severe damage
p53 Mutation
Cell division needs balance between proto-oncogenes and tumor supressors
Disrupts mitotic checkpoints so cancer can divide indefinitely
Withou p53, there’s no halting of cell cycle or signal for apoptosis
no DNA repair
p53 and cancer
p53 is mutatued or deleted in cancer and the pathway for it is disrupted
Allows cancer to evade checkpoints and apoptosis
It’s challenging to use the pathway for therapy but different strats are effective
p53 and cancer therapeutic challenges
targeting mutant p53 is identifying a binding site for it
It’s also in the nucleus mainly which is hard to access esp in cancer cells
There are many types of mutations which can be present in p53 for different molecules of it
p53 and cancer promising solution
Y220C mutant p53 PC14586
PC selectively binds to p53 Y220C and restores it
It creates a binding pocket for small inhibitors
Why does regulated cell death occur?
Normal part of cell life cycle
It’s an eqm where you gain and lose as a constant process
Helps in preventing cancer i guess by not having too little cell loss
Apoptosis in embryo hand/feet development
Even in early development, apoptosis takes place
In week 6, skin forms webbing between the digits
By week 11, the webbing disappears due to apoptosis
Proof of Apoptosis in Embryo Webbing (Mouse Paw)
Mouse paw embryo stained with a dye to detect apoptosis
Shown as yellow dots seen mostly where the webbing is
As webbing disappears, the bright spots do too
This is done through TUNEL assay
TUNEL Assay in Apoptosis Detection
Takes advantage of the nicks present in apoptosis cell’s DNA
dUTP can be incorporated into the nicks by enzymes
Enzyme: Terminal Deoxynucleotidyl Transferase
Flourescently-labelled dUPT can specifically detect cells in apoptosis
Apoptosis in Frog Metamorphosis
Tadpoles undergo metamorphosis to become a frog
The tail disappears as the cells are induced to undergo apoptosis
This is stimulated by the increase of the thyroid hormone in blood
Apoptosis in Human Nervous System Development
Half of the cells originally produced are required in normal brain development
The other half undergo apoptosis
Cells that haven’t achieved synaptic connections
Cells with faulty connections
Cells not having made contact with a target cell
Matches the number of nerve cells with the number of target cells
Inappropriate Cell Death Diseases
Alzheimers: Neurons in hippocampus and cerebral cortex die
Huntingtons: Neurons in striatum die
Parkinsons: Dopamine neurons in substantia nigra die
Duchenne Muscular Dystrophy: Muscle cells die
What are the 2 ways in which cells die
Necrosis
Apoptosis
Necrosis
Cell death through damage to exterior
Cells swell and release contents to surrounding tissue
Can lead to infection
Apoptosis
Programmed cell death that is regulated
Cells suicide in response to stress/damage or as a part of normal development
The debris isn’t released to damage cells nearby
The debris is contained and recycled
Apoptotic Pathway
Cell Execution: Kill the cell
Engulfment: Get rid of the body
Clearance: Destroying the evidence
Ultrastructural Features of Apoptosis (7)
Chromatin compacts and condenses
Nuclear envelope breaks down
Nucleus contents are fragmented and the DNA / proteins are degraded
Cytoplasm undergoes condensation as cellular components aggregate
Mitochondria is permeabilized and released into the cytosol
Cell membrane moves and changes shape to create blebs (protrusions)
Cell fragments create compartments with debris which will be phagocytized and recycled
C. elegans Apoptosis Model
They’re studied very much in detail
947 somatic cells have been identified in the adult worm
The lineage of them all is traced to a single cell undergoing rounds of division
131 cells undergo apoptosis
Apoptosis Genes Identified by C. elegans model
Done by assay for identifying mutations in genes
These genes are called “cell death genes” (ceds)
Mutation in ced-1: Allows apoptosis but not the associated phagocytosis
Mutation in ced-3: No apoptosis observed
Four essential genes:
ced-3
ced-4
ced-9
egl-1
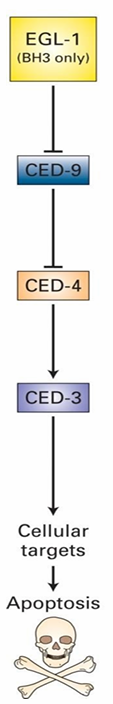
Mammalian Apoptotic Pathway
EGL-1 Homologs: Bid and Bim
CED-9 Homolog: Bcl-2
Bcl-2 controls Bak and Bax
CED-4/3 form a complex called the caspase holoenzyme
Protease targeting many different proteins for degradation
CED-3/4 mutations prevent death
ced-9 mutations make all cells die
Inhibits activation of caspase holoenzyme
Inhibits apoptosis in this was
EGL-1 signals apoptosis by inhibiting CED-9
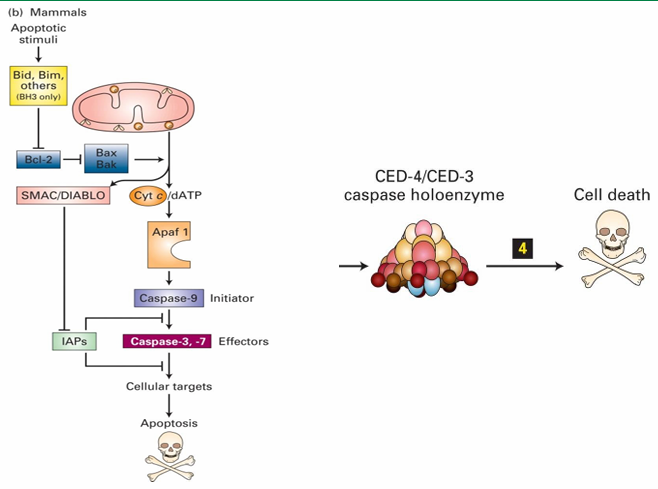
Caspase Holoenzyme
Apoptosome in mammalian cell
Contains direct homologues of C. elegans proteins
Apaf 1 = CED-4
Caspase-9 = CED-3
Protease activity of caspase holoenzyme leads to protein degradation and cell death
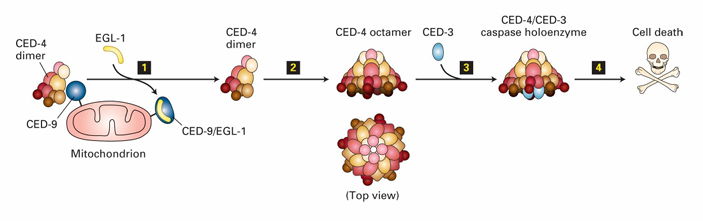
Activation of Caspase Holoenzyme (C. elegans)
In C. elegans
CED-9 inhibits apoptosis by binding to CED-4 dimers
Keeps them inactive
EGL-1 binding to CED-9 releases CED-4
CED-4 then join with CED-3 to form caspase holoenzyme
This leads to degradation of cytosolic and nuclear proteins
Mammalian CED-9 homologue
It’s Bcl-2
Normally anchored to outer membrane of mitochondria
Alters permeability of it
It maintains low permeability when present
When inactive, it forms pores associated with apoptosis
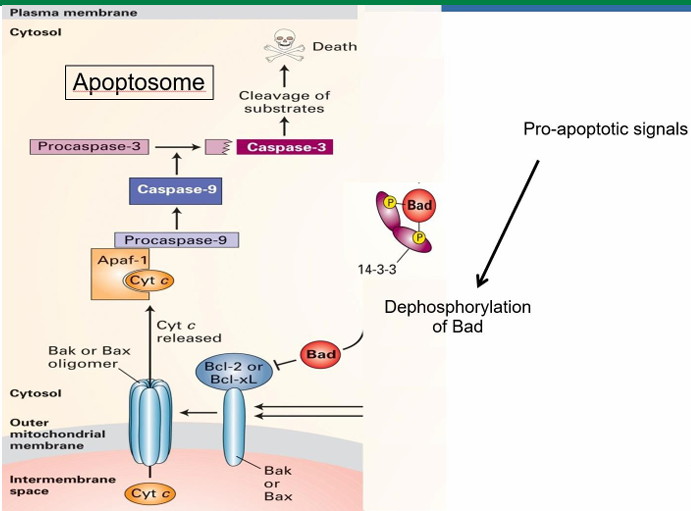
Bad: Apoptosis Signaling Pathway with Cytochrome C.
Mammalian cell apoptotic signal is called Bad
It’s inactive while phosphorylated and bound to 14-3-3
14-3-3 is a cytosolic adaptor protein
Signalling pathways allow dephosphorylation of Bad
It then releases from 14-3-3
It then binds to Bcl-2 on mitochondria
This activated Bcl-2 to allow for Bax to be activated
Bax aggregated into clusters in the membrane to make pores
Pores increase membrane permeability
Allows release of mitochondrial proteins into cytosol
This includes cytochrome C which is essential in forming mammalian apoptosome
Trophic Factors in Apoptosis Prevention
Trophic factors prevent apoptosis to keep the cell alive
They initiate a kinase cascade leading to phosphorylation of the Bad protein
When trophic factors are removed, Bad can be dephosphorylated
Dictyostelium discoideum slime mold
Eukaryote
Transitions from a unicellular amoeba to
multicellular slug
fruiting body
Aggregated amoeba form a slug
They then differentiate into 2 cell types
prestalk
prespore
The anterior end of the slug forms the stalk
Posterior end will form the spores of the fruiting body
Dictyostelium Vegetative Growth Phase
They feed on bacteria
when food is abundant, they divide by mitosis
This is vegetative growth phase
Dictyostelium aggregation
induced by starvation
happens in response to cAMP produced by starved cells
Aggregation forms the slug which moves to find suitable env
Dictyostelium in Suitable Env
When it finds a nutrient-rich env, it stops and begins to differentiate
Anterior cells form the stalk
Posterior cells form the fruiting body
The fruiting body contains spores with a hard cell wall
The spores will eventually germinate to form new single-celled amoebae
Dictyostelium receptor for cAMP + response
Transmembrane protein: G-protein coupled receptor (GCPR)
cAMP binds extracellularly to activate the receptor
Cells reorganize their intracellular actin cytoskeleton
This allows them to move towards the signal source
what enables the movement of Dictyostelium towards cAMP source
Dynamic filopodia extending outwards
Signaling initiated actin reorganization, including
nucleation
polymerization
depolymerization
All of this allows movement to occur
Disctyostelium movement with clathrin heavy chain mutation
Means cells can’t form vesicles needed for protein transport to cell membrane
cAMP is detected by transmembrane GPCR proteins
In the absence of clathrin, GPCR isn’t transported to cell surface
The cell is then unable to respond to the signal
There is no net movement towards the signal source
Neutrophils
WBC in our bodies
Can respond to signals made by bacteria invading our bodies
it’s irregularly shaped and is able to crawl to follow bacteria around RBCs
Signals enabling neutrophils to follow bacteria
Bacteria produced a protein signal containing
methionine
leucine
phenylalanine
Neutrophils have a receptor on the surface able to recognize this fMLP peptide
It’s a GPCR recetor
Signaling Definition
Signaling is the transmission of information from one cell to another that induces a change in behavior.
Signals are only useful if there’s a response to it
Principles of signal transduction pathway (STP)
Signaling cells produces and releases signaling molecules
Target cells have a receptor to bind to the signal
This activates the receptor to make a cascade of event
This cascade will interpret and transduce the signal to cause change in behaviour
Transcription
Cell movement / growth / differentiation
Many cells may be exposed to the signal, but only the target ones will have receptors to respond
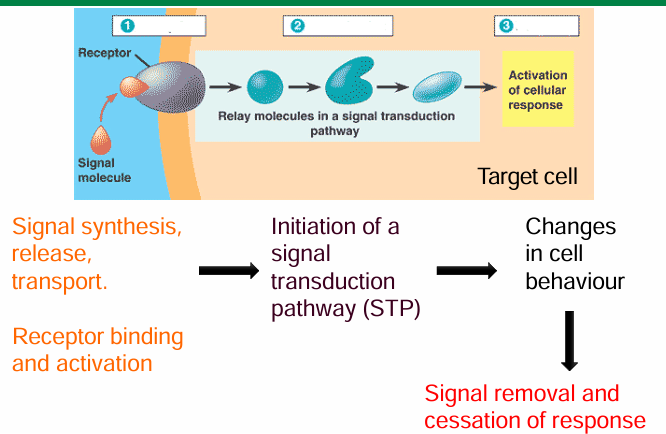
Specificity of signal-receptor interactions in signaling
They only bind if they have high molecular complementarity
It must be specific and high-affinity
Allows the interacting surfaces to come closer through essential amino acid residues being present
Induces conformational change in intracellular domain of receptor
This activates the STP to lead to a response
The 2 levels of signal response specificity
Specificity of signal for binding to receptor
Specificity if intracellular response mediated by STP
Same signal can activate different intracellular proteins of different cells
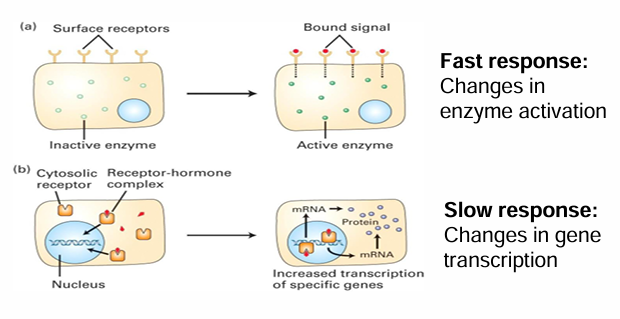
Fast Cellular Response
Extracellular signal binds to membrane-associated receptor
Activated cytosolic enzyme through a modification (ex. methylation)
Fast response bc cell can quickly respond by activating an already present protein in response
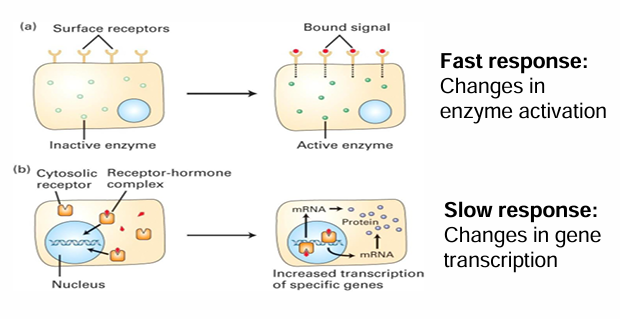
Slow Cellular Response
Binding of signal causes change in protein levels within cell
A soluble receptor is within the cytosol
The signal passes through the membrane to bind to it
Upon activation, the receptor is transported into the nucleas
It can directly/indirectly act as a transcriptional activator to produce mRNAs
This can increase protein levels within the cell
This is a slow response as it depends on many steps that take time
Measuring the Signaling (Enzyme Kinetics like Graph)
Affinity here measured similar to protein-ligand (pink)
100% means all the receptors are filled
x axis: signal conc
y axis: fraction of bound receptors
Kd: Dissociation constant
[signal] required to have half max binding
represents receptor-signal affinity
Measure of [signal] required to produce response (blue)
x axis: [signal]
y axis: fraction of cells responding
Max response can be measured, half can be calculated
[signal] needed to achieve half of response is less than to fill half of the receptors
Means signal amplification takes place
![<ul><li><p>Affinity here measured similar to protein-ligand (pink)</p><ul><li><p>100% means all the receptors are filled </p></li><li><p>x axis: signal conc</p></li><li><p>y axis: fraction of bound receptors</p></li><li><p>Kd: Dissociation constant </p><ul><li><p>[signal] required to have half max binding </p></li><li><p>represents receptor-signal affinity </p></li></ul></li></ul></li><li><p>Measure of [signal] required to produce response (blue)</p><ul><li><p>x axis: [signal]</p></li><li><p>y axis: fraction of cells responding </p></li><li><p>Max response can be measured, half can be calculated </p></li></ul></li><li><p>[signal] needed to achieve half of response is less than to fill half of the receptors </p><ul><li><p>Means signal amplification takes place </p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/da790d7f-b653-434e-a374-aedeb9b3a5d8.png)
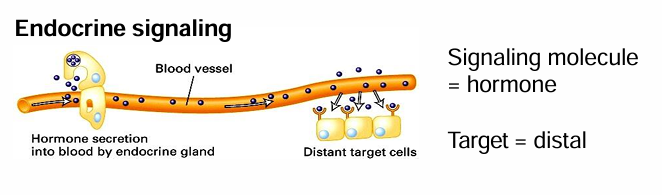
Endocrine (Secreted) Signaling
Signals are released into circulatory system
Cells throughout the body are exposed
Only cells with target receptors can respond
different cells of different tissues can respond at the same time
Common ex: Secreted Hormones
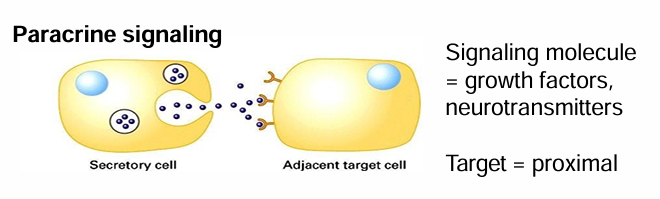
Paracrine Signaling
Secreted signals are released into extracellular space
They can diffuse into neighbouring cells
Signal and target cells are close
Common Ex. Growth factors and NTs
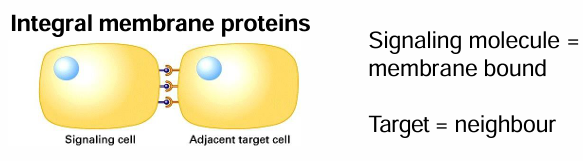
Proximal Signaling
Signaling and target cells are in direct contact
Signal and receptor proteins may be transmembrane proteins on different cells
So the interaction requires the cells to be attached by adhesion done by integral membrane proteins
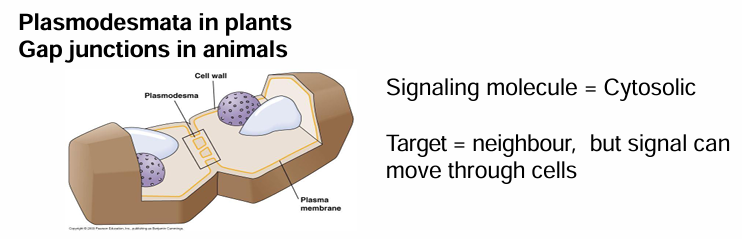
Cell Signaling by Cytosolic Messengers (Plasmodesmata / Gap Junctions)
Ex. In plants (plasmodesmata) and animals (gap junctions)
They have junctions between cells spanning the cell wall/membrance
This connects cytoplasm between the cells allowing messengers to move quickly
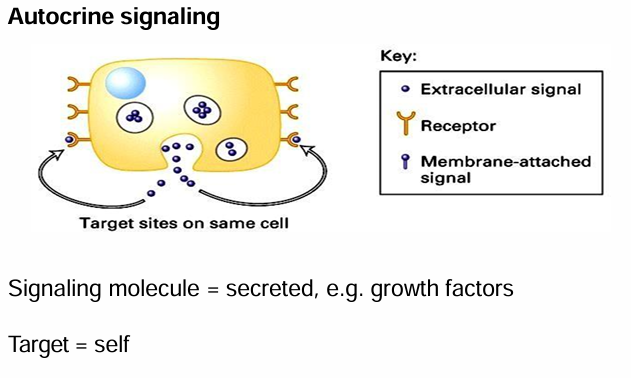
Autocrine Signaling
Cell communicating with itself
Signaling and target cell is the same
Cell produced secreted signal, and also carries receptors for it
Ex. Growth factors produced to induce cell division
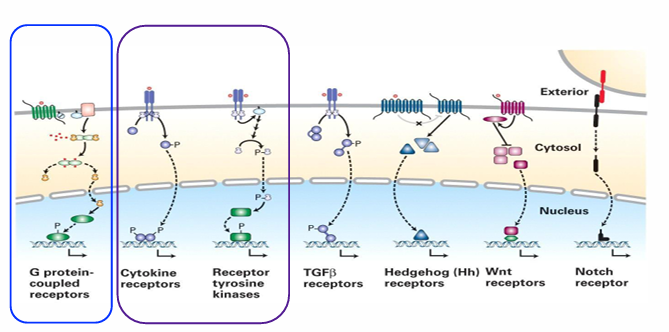
Classification of Cell-Surface Receptors
There are 7 total
We focus on 3 types
Cytokine receptors
Receptor-tyrosine kinases (RTKs)
G-protein coupled receptors (GPCRs)
Extravasation
The movement of WBC from blood stream to surrounding tissue
5-step process initiated by a signal created by infection
Why are transient (temporary) cell adhesions important, and when do they occur?
Not all cell adhesions are permanent — some are temporary to allow movement.
Transient adhesions are essential for:
Cell migration across extracellular surfaces.
Cell movement during embryogenesis.
These connections form and break repeatedly, enabling cells to travel where needed.
How do leukocytes use transient adhesion during an immune response?
Leukocytes must exit blood vessels to reach sites of infection or injury.
This extravasation relies on a sequence of temporary adhesive interactions with endothelial cells
Normally, adhesion between endothelial cells prevents blood leakage
During an immune response, leukocytes temporarily attach and cross the vessel wall to enter tissues.
What are the 3 families of WBCs / Leukocytes
Granulocytes: Neutrophils
Monocytes: Macrophages
Lymphocytes: T and B cells
Granulocytes
Target pathogens
Include neutrophils, eosinophils, and basophils
Neutrophils
Most common granulocyte
Primarily targets bacteria infections
One of the first cells to respond to trauma
Capable of extravasation
Monocytes
They differentiate into microphages
They engulf invading bacteria or dead cells through phagocytosis
Capable of extravasation
Lymphocytes
Include NK (natural killer) cells
Lyse virally infected cells and tumour cells
Include T and B cells
Produce antibodies as immune response
Can undergo extravasation
What are the five steps of extravasation
capture
rolling
slow-rolling
firm adhesion
transmigration
Extravasation: Step 1
Capture (Using Neutrophil Ex)
This is the transient association between the neutrophil and the apical surface of endothelial cell
They’re still being pushed by bloodflow but slower
The cells roll along the surface of endothelial cells
Extravasation: Step 2 / 3
Rolling / Slow Rolling
Since the transient associations are slowing the neutrophil, it rolls along the surface
The rate slows down as # of associations increase
This leads to firm adhesion
Extravasation: Step 4
Firm adhesion
Occurs with stronger attachment of neutrophil with endothelial cells
This is accompanied by changes allowing the WBC to break connections b/w endothelial cells
This allows migration along the cell surface to outside the blood vessel
Extravasation: Step 5
Transmigration
The seperation of endothelial cells allow the neutrophil to migrate out of the blood vessel
Causes swelling as transmigration occurs
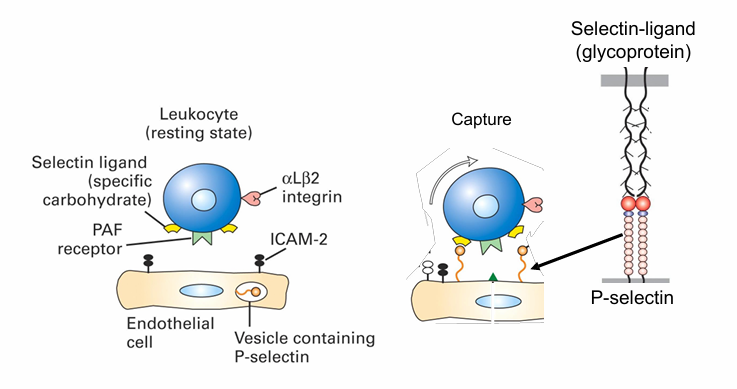
Extravasation Capture Mechanism
Cytokines (e.g., TNF-α) are released at the infection site
They signal endothelial cells of blood vessels.
This signal (received at the basal surface) triggers endothelial cells to move P-selectins from secretory vesicles to their apical surface.
P-selectins on the endothelial surface then bind to selectin-specific glycoprotein ligands on neutrophils
This captures them from the bloodstream and initiates the immune response.
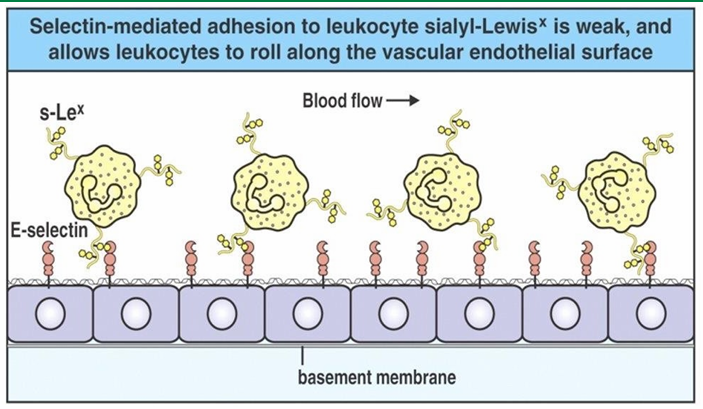
Extravasation Rolling Mechanism
Adhesion of neutrophil to endothelial cells slow movement
Eventually, they start rolling along the walls
This involves them being pushed over the surface while establishing and losing transient connections
Extravasation Slow-Rolling Mechanism
Density of selectins on endothelial cells inc closer to site of infection
Many endothelial cells are displayed P and E selectin here
The inc associations between selectins and the ligands on neutrophils flows their movement
They are no undergoing slow-rolling
Extravasation Firm Adhesion Mechanism
Slow rolling lets new interactions form between neutrophils and endothelial cells.
PAF (platelet activating factor) on endothelial cells binds to the PAF receptor on neutrophils (a
Ex. receptors CXCR1 and CXCR2
This interaction occurs only during slow rolling and activates a signal transduction pathway inside the neutrophil.
The signal activates integrin adhesion molecules on the neutrophil, enabling them to bind ICAMs on endothelial cells.
This binding slows the neutrophil further, leading to firm adhesion (tight binding) to the vessel wall.
Integrin Protein Structure (Extravasation Firm Adhesion)
Inactive integrin (dimeric) has its propeller and β-A domains folded down, preventing ligand binding.
PAF signaling triggers a conformational change, activating the integrin so it can bind ICAMs on endothelial cells.
Integrin–ICAM binding is much stronger than selectin interactions, resulting in firm adhesion of the neutrophil.
Activation also initiates actin cytoskeleton reorganization, preparing the neutrophil for cell migration out of the blood vessel.
Extravasation Transmigration Mechanism
The neutrophil has stopped at the site of infection
It can migrate b/w the endothelial cells
The connections b/w them are broken by enzymes produced by transmigrating neutrophil
Progressive Activation of Extravasation
Selectins are activated first
Mediates capture, rolling, and slow-rolling
Signalling pathways activate integrins
Mediates firm adhesion
Allows transmigration
H.V. Wilson Sponge Experiment
First demonstrated the ability of cells to recognize and adhere to one another
Used the cells of 2 sponge species
Their indiv cells were seperated using a fine mesh
The cells were then mixed together
Overtime, the cells from the same species were able to recognize and associate back together
Cells from diff species didn’t associate
Johannes Holtfreter: Frog Embryo Experiment
Showed cell recognition and adhesion using frog embryos
Took cells from 2 different developmental germ layers and seperated indiv cells
Similar tissue recognized eachother and associated
The associations mimicked original embryo organization