Colloids SOTAs, Glycocalyx MDRS, Lactate MDRS, Fluid Therapy MDRS
1/181
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
182 Terms
Advantages of Dextran
Macromolecular polysaccharide produced from lactic-acid bacterial fermentation of sucrose
Neutral, high-molecular weight (Mw) glucopolysaccharides which require hydrolysis into lower Mw between 40 and 70 kDa, suitable for intravenous infusion
Major Adverse Effects of Dextran
Anaphylactic reactions
Result of naturally occurring dextran-reactive antibodies, causing a type III or immune complex reaction
Risk of severe reactions can be reduced by prior administration of a monovalent hapten dextran, which is thought to bind dextran-reactive antibodies and form inactive complexes
Osmotic kidney failure with hypertonic dextran preparations
AKI has most often been reported following administration of 10% dextran 40 solutions
Most published reports suggest contributing factors such as age, repeated infusion of large quantities, and arteritis
Impaired coagulation
Related to a decrease in vWf/FVIII-complex, weaker clot strength, and platelet dysfunction
Advantages of Modern Gelatin Products
Inexpensive
Long shelf life
Disadvantages of Modern Gelatin Products
The low mean Mw results in rapid renal excretion and the volume expanding effect of 70-80% is very short lived (2-3 hours)
Increased diuresis
AKI
Increased blood viscosity
High rate of anaphylactic reactions compared to dextran and HES
EMA Recommendations for HES Use
The second European Medicines Agency (EMA) Pharmacovigilance Risk Assessment Committee (PRAC) concluded that HES solutions should no longer be used to treat patients with sepsis or burn injuries or in critically ill patients because of an increased risk of AKI and mortality
The final decision letter posed on the EMA website stated that HES solutions may be used to treat hypovolemia caused by acute blood loss, where treatment with crystalloids alone is not sufficient, and that to minimize potential risks, HES solutions should not be used for more than 24 hours and kidney function should be monitored
What are HES solutions characterized based on?
Their raw material
Concentration
Mean molecular weight
Molar substitution
Ratio of C2/C6 hydroxylation
Carrier solution
What does the percentage concentration of HES solutions effect?
The percentage concentration of HES solutions mainly affects the initial immediate volume effect after administration
Approximately 145% for 10% HES solutions compared to 100% for 6% HES solutions
What does the molecular weight of HES effect?
The oncotic effect depends on the number of oncotically active particles and not so much the concentration
A product with molecules of low Mw is likely to exert greater COP at similar concentrations than products with molecules of high MW
Molecular Weight of First Generation HES
>400 kDa
Molecular Weight of Second Generation HES
200-400 kDa
Molecular Weight of Third Generation HES
<200 KdA
What does the rate of breakdown of larger molecules of HES depend on?
Molar substitution
Ratio of C2/C6
What is the effect of molar substitution of HES?
Natural amylopectin is chemically modified by hydroxyethylation at the glucose subunits C2, C3, or C6 to increase solubility and inhibit degradation by a-amylase
The average number of hydroxyethyl residues per glucose subunit defines the MS, with higher substitution associated with delayed enzymatic degradation
Hetastarch Molar Substitution
0.7
Hexastarch Molar Substitution
0.6
Pentastarch Molar Substitution
0.5
Tetrastarch Molar Substitution
0.4
Effect of C2/C6 Ratios of HES
Higher plasma concentrations and a longer duration of the oncotic effect was found with higher C2/C6 ratios due to slower enzymatic degradation
Effects of Carrier Solution of HES
Significantly lower serum chloride concentrations and less acidosis were found after administration of balanced electrolyte and buffered carrier solutions
Balanced HES resuscitation was shown to be superior in terms of improved renal perfusion
Plasma Colloid Oncotic Pressure
The plasma colloid oncotic pressure (pic) opposes fluid filtration out of the vascular space
Depends largely on the concentration of albumin
Plasma Hydrostatic Pressure
Plasma hydrostatic pressure (Pc) is the driving force for fluid filtration out of the vascular space
Depends on arterial blood pressure and vascular resistance
Interstitial Colloid Oncotic Pressure
Interstitial colloid oncotic pressure (pit) contributes to fluid filtration out of the vessel
Varies between tissues depending on interstitial albumin content, with higher pit in the lungs compared to skeletal muscle or subcutaneous tissue
Transcapillary Fluid Shifts Based on the Starling Equation
Based on the Starling equation, a constant outward movement of fluid occurs on the arterial side of the capillary while a slight inward movement occurs on the venous side due to differences in hydrostatic pressure
Excess interstitial fluid, which is not reabsorbed on the venous side, returns to the circulation via lymphatic vessels
Any imbalance between these forces or changes to the coefficient of filtration, such as occurs with hypoalbuminemia or capillary leak, results in edema formation
Revised Starling Model
A revised Starling model was proposed, in which, instead of fluid filtration on one end and resorption on the other end of a capillary, fluid exit occurs over the entire length of the capillary
In this model, a COP difference exists between the intravascular space and a small, almost protein-free, subglycocalyx zone, which serves as the principal determinant of fluid flux from the vasculature
The glycocalyx is a web of membrane-bound glycoproteins and proteoglycans carrying negatively charged side chains, which covers the entire endothelial surface
Outwardly shifting proteins are bound in this filter-like structure, which becomes loaded with plasma proteins
This layer together with the subglycocalyx and bound proteins form the endothelial surface layer (ESL) which is the noncirculating portion of the intravascular space
~700-1000 mL in adult humans
COP Difference Across the Glycocalyx
The COP difference across the glycocalyx opposes fluid exit and maintains vascular integrity
Almost no absorption occurs from the interstitium into the intravascular space, even at low capillary pressures
This nonlinear relationship between the capillary pressure and the movement of fluid through the capillary wall is achieved only where the capillary is in steady state, adapted to low pressure
A linear relationship and reabsorption from the interstitium does occur when the capillary pressure is lowered abruptly (i.e. acute hypovolemia) in a so-called transient state
Functions of the Glycocalyx
The glycocalyx has a transmural vascular barrier function, anti-inflammatory properties, and impedes blood cells from direct contact with the endothelium
What can degrade the glycocalyx?
Inflammation, sepsis, ischemia/reperfusion, increased atrial natriuretic peptide, and diabetes are some of the conditions leading to a reduction of the endothelial glycocalyx layer, mainly through shedding of glycosaminoglycans, with resultant platelet aggregation, leukocyte adhesion, increased vascular permeability, and edema formation
Iatrogenic hypervolemia leads to degradation of the endothelial glycocalyx
During acute hypervolemia, atrial natriuretic peptide is secreted which causes rapid shedding of the glycocalyx with a subsequent increase in permeability
Volume Effect of Colloids vs Crystalloids
Approximately 3-4 times more crystalloids than colloids are required to achieve an equivalent plasma volume expansion
Crystalloid solutions equilibrate rapidly between the intravascular and interstitial fluid spaces while the large molecules of colloids may absorb to the glycocalyx layer and restrict ultrafiltration
Colloids remain for several hours within the intravascular space as opposed to only 30-60 minutes for most crystalloid solutions
Only occurs in vivo with an intact glycocalyx layer, which may be destroyed in conditions leading to SIRS, such as trauma, surgery, severe illness, and sepsis, as well as in hypervolemia
Other studies found that HES is likely superior to crystalloids in conditions, such as hypovolemia, in which the glycocalyx remains intact but that the volume expanding effects of HES may be no greater than crystalloids when the glycocalyx is damaged
Plasma volume can be divided into circulating and noncirculating (ESL) portions so the volume effect exerted by HES might be due more to a decrease in the noncirculating portion rather than an oncotic pull from the interstitium itself, with the "dehydration" of the glycocalyx leading to its damage
HES Plugs the Leaks in the Vascular Endothelium
HES does have properties that attenuate capillary leakage
The exact mechanism is unclear
The effects of HES on vascular permeability in patients affected by spontaneous disease remains unclear
HES Exerts an Anti-Inflammatory Effect
Results of studies point consistently toward an anti-inflammatory effect of HES
Another set of studies demonstrated an overall decrease in activity of inflammatory cells in the presence of HES
HES Has Pulmonary Protective Effects
Most studies report a positive effect of HES in decreasing pulmonary permeability
The majority demonstrated a general ability of HES to maintain intravascular fluid and thereby mitigate pulmonary dysfunction compared to crystalloids
These results may have been affected by the much larger volumes of crystalloids used, exacerbating pulmonary leakage
When noncolloidal low-volume resuscitation agents, such as hypertonic saline, were included, they were found to produce similar pulmonary protective effects as HES
HES Increases COP and Prevents a Decrease in COP
The plasma colloid oncotic pressure depends on the plasma concentration of albumin (80%), globulins, and fibrinogen
Due to the water binding capacity of albumin, it is responsible for maintaining fluid within the vascular space and a marked decrease leads to edema formation
The severity of peripheral edema was found to correlate with plasma albumin concentrations and COP in critically ill dogs
Studies found that multiple doses of HES are necessary to achieve a long-lasting effect on COP
HES Has an Improved Risk/Benefit Profile
The major concerns in the use of any colloid include anaphylactic reactions, coagulopathies, tissue accumulation, renal failure, and increased mortality
Studies suggest that when it comes to choosing a colloidal product, tetrastarch may be the least detrimental, but this does not necessarily imply that is should be chosen before crystalloids
One major difference from findings in people is that synthetic colloids are associated with a several fold increased incidence of anaphylactoid reactions in people compared with albumin, but HES-associated anaphylaxis appears extremely rare in dogs and cats
In contrast, severe adverse effects including death have been reported in both healthy and critically ill dogs following administration of human albumin
HES may be considered safer than albumin in veterinary medicine as long as species-specific albumin is unavailable
HES for Perioperative Fluid Therapy
Vasodilation and hypotension due to anesthetic agents, ongoing insensible losses, as well as procedure-related fluid losses justify the use of perioperative fluid therapy
Conventional perioperative fluid therapy in people consisted of large volumes of crystalloids, often associated with perioperative fluid overload and gain in body weight
Sequela of perioperative fluid overload include cardiopulmonary dysfunction, reduced intestinal motility, dehiscence of small bowel anastomoses, abdominal compartment syndrome, increased extravascular lung water, decreased pulmonary function, impaired wound healing, and impaired hemostasis
Studies in humans have had conflicting results
Studies evaluating the perioperative efficacy of HES in animals have generally found HES superior to crystalloids but have been restricted to the experimental field
The use of HES in preanesthetic volume loading before spinal anesthesia to prevent sympathetic-blockade hypotension has been evaluated
Studies show conflicting results
A general recommendation as to the use of perioperative HES in small animals is difficult based on the few studies performed
Further RCTs are necessary but given current concerns, the benefit for perioperative patients may be questionable
HES for Absolute or Relative Hypovolemia
Potential advantages of HES over crystalloids in the treatment of shock are a more rapid restoration of intravascular volume, increase in COP, longer-lasting volume effects, and diminished extravasation
Recent studies and reviews in people showed that colloids were safe and efficacious for initial fluid resuscitation in trauma victims
In recent veterinary reviews, colloids have been recommended for hypovolemic (trauma, severe loss of plasma volume) and distributive shock, but this is mostly based on human literature
Fluid therapy to increase intravascular volume without exacerbating vascular leakage is desired to treat hypovolemia and organ impairment due to edema formation in patients with sepsis or ischemia-reperfusion injury
Recent RCTs in people found increased mortality and need for RRT in patients receiving HES versus crystalloids
A Cochrane meta-analysis on the effectiveness and safety of colloids versus crystalloids in critically ill patients and between different types of colloids in acute hypovolemia concluded that there is no evidence from randomized controlled trials that resuscitation with colloids reduces the risk of death, compared to resuscitation with crystalloids in patients with trauma, burns, or following surgery and there is no evidence that one colloid solution is more effective or safer than any other
The Surviving Sepsis Campaign advised against the use of HES in its 2012 edition
HES for Hypoalbuminemia and Normalization of COP
The use of HES appears to have beneficial effects in hypoalbuminemia by increasing COP and reducing the severity of peripheral edema in dogs but this requires an intact glycocalyx
Both albumin and HES failed to prevent fluid leakage into the interstitium in studies following glycocalyx destruction
Albumin generated a better vascular barrier than HES, with levels of transudate formation similar to those observed after HES administration with an intact glycocalyx
In this same series of studies, similar transudate formation is shown with use of crystalloid solutions in both conditions of intact and damaged glycocalyx, suggesting that without colloid, the glycocalyx has no barrier properties
The glycocalyx and its bound proteins act as an interdependent double barrier for vascular permeability
Attempting to raise COP in conditions of a damaged glycocalyx might not only be futile but also injudicious, leading to dangerous extravasation of colloid and worsening edema
Given that the COP gradient opposing transudation is determined by the ESL and not intravascular COP, normalization of COP should no longer be considered a necessary goal of fluid therapy
Increasing COP may suppress albumin synthesis, further increasing the risk of fluid extravasation
Availability of blood products and albumin is generally inferior in veterinary medicine and human albumin is dangerous in dogs and is not recommended
HES may be the best option in veterinary medicine for patients with acute hypoalbuminemia when blood products are unavailable
COP Paradox
The ability of albumin (and other colloids) to “seal” the vascular endothelium and reduce extravasation independent of its effect on COP
HES for Hemodialysis and Continuous RRT
Used during hemodialysis as a priming solution
The small body weight and circulating blood volume of human pediatric patients and small animals results in a relatively large priming volume in RRT units
Priming of the hemofiltration circuit can lead to a dangerous decrease in circulating blood volume
Artificial colloids have been used to mitigate hypotension during priming in RRT units and for apheresis
Adverse Effects of HES
Tissue storage
AKI
Hemorrhage
Anaphylactoid reactions
Pruritis
Development of HES preparations with lower Mw and MS has been claimed to minimize these risks
Theories to Explain the Mechanism of Impaired Coagulation with HES
Dilution of clotting factors and platelets leading to dilutional coagulopathy
Hemodilution following HES administration in people was found to result in decreased concentrations of factor VIII/vWF complex, vWF-ristocetin cofactor, and vWF antigen, as well as accelerated elimination of factor VIII/vWF complexes and diminished vWF-mediated rolling and adhesion of platelets to subendothelial collagen
Platelet dysfunction following HES administration is described to be caused by decreased platelet numbers due to dilutional effects, colloid-osmotic shrinkage of platelets, and increased platelet degradation
Interaction with factor VIII/von Willebrand factor (vWF) complex, fibrinogen polymerization, and glycoprotein receptor GIIbIIIa (GPIIbIIIa) on the surface of activated platelets
Coating of platelets with HES leads to decreased expression and activation of the surface receptor GPIIb IIIa which binds vWF and fibrinogen and plays a key role in platelet adhesion and aggregation
HES exerts a pro-fibrinolytic action as its incorporation in clots leads to accelerated conversion of fibrinogen to fibrin, resulting in a friable clot, as well as reduced clot firmness due to decreased interaction between activated factor XIII and fibrin
What does the degree of hemostatic impairment due to HES depend on?
Degree of hemostatic impairment due to HES depends on physicochemical and pharmacokinetic properties
A high C2/C6 ratio or high MS may delay enzymatic degradation and these properties enhance intravascular HES accumulation, resulting in increased coagulation impairment and plasma viscosity
Despite improved physicochemical properties of HES 130/0.4, a systematic review found that it leads to hypocoagulability at low concentrations and in a dose-dependent fashion
Impaired Coagulation Due to HES
Overall findings suggest that similar effects on coagulation are observed in dogs and people
The data so far gathered suggests that all HES products potentially affect coagulation and this is likely dose dependent (upwards of 10 mL/kg), although the duration and severity may be less with tetrastarch than penta- or hetastarch preparations
Tissue Storage of HES
Intracellular storage of HES molecules may lead to organ dysfunction
Organs involved include the bone marrow, intestine, kidney, liver, lung, lymph nodes, monocyte-macrophage system, muscle, pancreas, reticular-endothelial cells, skin, and spleen
Uptake seems to be rapid
In part, HES is split enzymatically in the plasma by a-amylase, followed by urinary excretion
Also metabolized by phagocytosis by immune cells and subsequent storage in intracellular vacuoles
HES molecules are also taken up by a variety of nonphagocytic cells including epithelial cells, endothelial cells, hepatocytes, keratinocytes, and Schwann cells by pinocytosis
HES is absorbed in the proximal tubular epithelial cells in the kidney during the excretion process
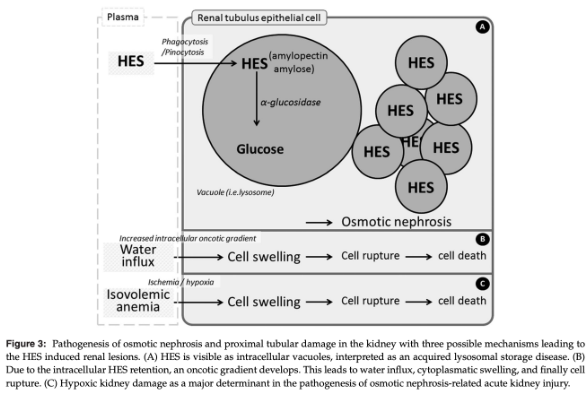
Phagocytized HES molecules cause an acquired lysosomal storage disease, evident morphologically as foamy macrophages
Intracellular HES accumulation can persist for long periods of time and is a dose-dependent and time-related process
Modern tetrastarch solutions, designed to have decreased tissue uptake are accumulated to a greater extent than older starch preparations
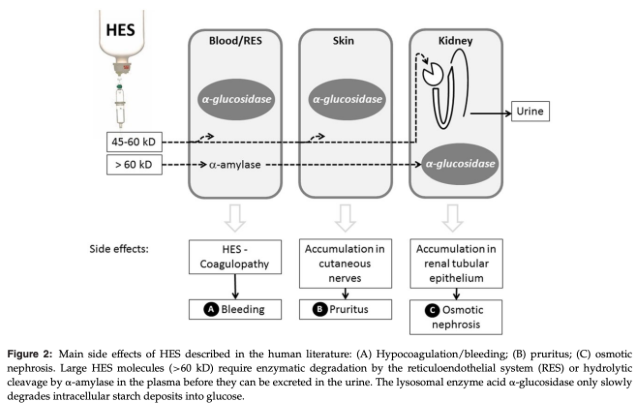
What are the lesions described in association with renal HES storage characterized as?
Osmotic nephrosis or osmotic nephrosis-like lesions
Osmotic Nephrosis
Histomorphological pattern with vacuolization and swelling of the renal proximal tubular cells
Distal tubules and collecting ducts are usually not affected
Changes can last for years, but are in general reversible and functional/structural recovery takes place sometimes after discontinuation of the agent
Liver Storage of HES
Several studies in humans and animals describe HES associated hepatic insufficiency, but a relationship between the vacuolar storage of HES in the liver and clinical signs is unproven
Repeated administration of HES may account for severe portal hypertension, liver failure, and sepsis, particularly in patients with chronic liver disease
In the CHEST study, the incidence of new hepatic organ failure was significantly higher in the HES group than in the saline group in ICU patients
Skin Storage and Pruritis with HES
Deposition of HES in the skin, detected as intracytoplasmic vacuoles in dermal macrophages, endoneural connective tissue cells, endothelial cells, Langerhans' cells, perineural cells, and Schwann cells is though to be responsible for persistent pruritis in people
HES can appear in skin cell vacuoles as soon as 90 minutes after a single dose of 30 g, with symptoms starting during or after treatment and persisting for long periods of time
Mediators other than histamine must be responsible as no histamine release from mast cells has been detected morphologically
The degree of vacuolization in the skin was not related to the type of HES
The degree of macrophage HES storage correlated with both the cumulative effect and the time point of the investigation in sheep
AKI with HES
The recent Cochrane review concluded that "all HES products increase the risk for AKI and RRT in all patient populations and a safe volume of any HES solution has yet to be determined
HES and Mortality
In a meta-analysis, patients receiving HES were found to have a slight increased risk ratio (1.09) of death compared to patients receiving other resuscitation fluids
Recent other meta-analyses and large RCTs came to similar conclusions
In the VISEP trial a trend toward higher 90 day mortality in septic patients was observed in patients receiving 10% pentastarch compared with LRS
Mortality was significantly increased among patients receiving high doses of HES compared to those receiving lower doses
The trial was stopped early for safety reasons, including a higher rate of severe hypoglycemia, serious adverse events in the intensive-insulin therapy group compared to the conventional therapy group, and higher rates of AKI and RRT in the pentastarch group compared to the LRS group
The 6S trial found higher mortality in patients receiving HES compared to Ringer's acetate
In both the VISEP and 6S trial, the separation of the survival curves occurred around day 20 in both studies, suggesting that increased mortality associated with HES is a late finding
Both trials also demonstrated that HES was associated with impaired kidney function and an increased need of RRT, which further contributes to higher mortality
The CHEST trial did not find a significant difference in mortality in critically ill patients receiving HES compared to those receiving saline, although the HES group did have a greater need of RRT
Factors Implicated in Contributing to Apparent HES-Associated Mortality
Impaired renal function
Increased risk of hemorrhage
Toxic effects of HES deposition in tissues
Recommendations for HES Use
The current PRAC recommendation (2013) does not recommend the use of HES in septic, burn, and critically-ill patients due to an increased risk of AKI and mortality and because no clear benefit has been demonstrated
Severe coagulopathies or impaired kidney function, including a need for RRT, are considered contraindications for the use of HES
The recommendation for use of HES in trauma and elective surgery was that HES can continue to be used in patients with acute hypovolemia due to acute blood loss, but only for a period of 24 hours, at the lowest dose possible, and for the shortest duration possible, and that kidney function should be monitored for up to 90 days
The Surviving Sepsis Campaign recommends against the use of HES products for fluid resuscitation of severe sepsis and septic shock and suggests the use of albumin in patients requiring colloids (2012)
An advisory note was issued by the AVA in 2013 informing that HES can still be used in veterinary patients, but suggesting cautious decision making in its administration
No other official organization has provided recommendations for the use of HES in small animals
The safety or effectiveness of HES products have never been approved in animals by the FDA and are therefore considered unapproved drug use
No safe dose or treatment duration has been established in small animals and previous recommendations were largely based on extrapolation from early reports in people or empirical use
Early Goal Directed Therapy
Early goal directed therapy has demonstrated an outcome benefit in human septic shock patients and likely imparts a survival advantage in other patient populations
Directs resuscitation toward restoration of an effective circulating blood volume and adequate end-organ perfusion
Expansion of the intravascular space with exogenous fluids is a first line therapeutic intervention to restore perfusion and a fundamental component of early goal directed therapy
Crystalloids
Salt solutions that convey negligible oncotic pressure across the endothelial barrier
Dissemination of Crystalloids Following Administration
Infusion results in rapid dissemination into the entire extracellular fluid space, with 60-80% of the administered volume redistributed out of the vascular space and into the interstitium within 30-60 minutes
Substantial fluid volumes are required to adequately expand the intravascular space, correct hypovolemia, and restore end-organ perfusion in circulatory failure
Normal Saline (0.9%)
Isotonic but not a physiologic solution as it contains a significantly higher chloride concentration and lower SID when compared to plasma
Resuscitation of critically ill patients with large volumes of normal saline may result in hypernatremia, hyperchloremia, and contribute to the development of a hyperchloremic metabolic acidosis
Risk of hyperchloremia-induced AKI
Postulated to result from high chloride levels inducing renal vasoconstriction, decreased glomerular filtration, and subsequent ischemic renal tubular damage
Isotonic Crystalloid Solutions
E.g. lactate Ringer's and Normosol-R
More physiologic than saline, containing a myriad of electrolytes as well as a buffer, typically lactate or acetate
The buffer additive requires metabolism prior to excretion
Theoretical contraindications to use of these solutions exist in particular disease states such as hepatic dysfunction
Human clinical trials have demonstrated that administration of a balanced salt solution over normal saline in surgical patients and critically ill patients was associated with a significant decrease in the rate of major complications, including postoperative infection, RRT, and need for blood transfusion
A recent large, retrospective study in critically ill adults with sepsis found that resuscitation with balanced solutions as opposed to normal saline, was associated with a lower risk of in-hospital mortality
Results of Aggressive Resuscitation with Crystalloids
The net positive fluid balance that occurs secondary to aggressive resuscitation with crystalloids is associated with worsened patient outcome
Expansion of the total extracellular space causes direct damage to the architecture of the endothelial surface layer (ESL), culminating in impaired capillary exchange
Progressive capillary leak, tissue edema, and hypoxia ensue with subsequent detrimental effects on cardiovascular, pulmonary, gastrointestinal, and renal function
Resuscitation Injury with Crystalloids
Isotonic crystalloid administration induces a proinflammatory state, with augmented inflammatory cytokine production and endothelial cell activation
Exaggerated inflammatory response exacerbates the effects of fluid overload, generating worsened edema
Other forms of resuscitation injury
Development of dilutional coagulopathy
Compartment syndromes
Impaired tissue healing
At the cellular level, aggressive volume resuscitation incites cellular swelling, leading to cytosolic acidification, altered intracellular protein function and signaling pathways, and ultimately impaired cell activity
Colloidal Solutions for Resuscitation
Selection of a resuscitation fluid with a volume of distribution limited to the intravascular space should minimize tissue edema formation
Both natural and synthetic colloids carry the theoretical benefit of a volume-sparing effect with a decreased risk of inducing a positive fluid balance
These solutions contain large molecules that cannot cross an intact vascular barrier
According to Starling's model of transcapillary fluid exchange, solutions hyperoncotic to plasma may generate a larger oncotic gradient across the endothelial barrier
This gradient functions to impede fluid movement to the interstitium and further augment the intravascular volume
Benefits of Natural Colloids
Albumin-containing fluids are thought to have benefits beyond the colloidal and volume-sparing effects, including roles in serum drug and hormone binding, protection from oxidative damage, anticoagulant effects, and maintenance of vascular integrity
Human Serum Albumin
The most frequently utilized natural colloid in human critical care is 4% human serum albumin (HSA), an isooncotic solution of purified albumin
Human serum albumin has been utilized in clinical veterinary patients
Several veterinary reports have demonstrated severe, even fatal type III hypersensitivity reactions in dogs treated with HAS
Severe hypersensitivity reactions were thought to be limited to HSA administration in healthy dogs due to decreased ability of clinically ill dogs to mount an immune response
Powell et al recently published confirmed type III hypersensitivity reactions in 2 ill dogs treated with HSA
There is a significant risk with repeat administration, as high levels of anti-HSA antibodies are produced in dogs within weeks of administration
The administration of HSA solutions to veterinary patients is controversial and likely not indicated for resuscitation purposes
May have a role in treatment of specific populations of severely hypoalbuminemic veterinary patients
Natural Colloid for Veterinary Patients
There is no equivalent, commercially available, species-specific, isooncotic natural colloid for use in veterinary patients
Lyophilized canine-specific albumin is sporadically available
In a small clinical trial, canine-specific albumin administration in 7 dogs with septic peritonitis resulted in increased albumin concentrations, colloid osmotic pressure (COP), and diastolic blood pressure 2 hours post-transfusion compared with a control group
Frozen Plasma and Fresh Frozen Plasma
Plasma products such as frozen plasma (FP) and fresh frozen plasma (FFP) are isooncotic natural colloids that can be incorporated into resuscitation algorithms
Used primarily for patients with acute hemorrhage or coagulopathy
Provide albumin, coagulation factors, and immunoglobulins, among other plasma components
Carries the risk of transfusion related adverse events including febrile nonhemolytic transfusion reactions (FNHTR), transfusion related acute lung injury (TRALI), immunomodulation, and potential pathogen transmission
Large volumes of plasma are required to affect plasma COP, especially in the face of ongoing protein loss (e.g. 40 mL/kg of plasma is required to increase serum albumin concentration by 1 g/dL)
Limited availability and expense must be taken into account
Oncotic Formulations of HES
Hydroxyethyl starch solutions are available in an isooncotic formulation (6%) and hyperoncotic formulation (10%) which has gone out of favor due to safety concerns
What determines degradation of HES molecules and HES half-life?
Serum amylase activity as well as the molecular structure of HES
What does the rate of HES degradation by serum amylase depend on?
The product molecular weight, molar substitution, and C2/C6 substitution ratio
MS and C2/C6 ratio are the most influential features
Effect of Molecular Weight on Ongoing Colloidal Support
Continuous degradation of larger HES molecules into small ones functions as a reservoir for colloidal replenishment
Larger MW solutions have a longer overall plasma half-life and greater capacity for ongoing oncotic support
Molar Substitution of HES
Molar substitution refers to the number of hydroxyethyl groups substituted per 100 available anhydrous glucose residue binding sites
E.g. a MS of 0.5 indicates that 50% of available binding sites are occupied
MS also dictates starch classification
0.4 MS is a tetrastarch, 0.5 is a pentastarch
MS is positively correlated with HES plasma half-life and retarded degradation, with plasma clearance of tetrastarches at least 20 times higher than that of hetastarch or pentastarch
C2/C6 Substitution
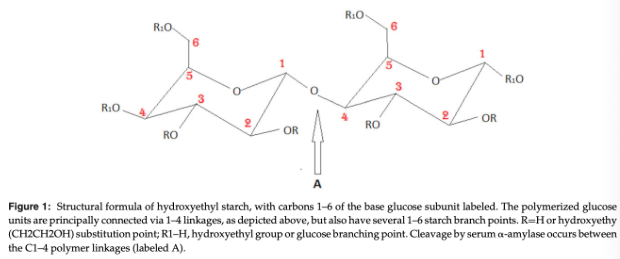
Hydroxyethyl groups are substituted on the C2, C3, and C6 carbons of the base glucose monomer
The pattern of substitution among these 3 sites affects amylase affinity and ultimately degradation efficacy
Substitution on the C2 position in particular leads to allosteric inhibition of the cleavage of the HES molecule by plasma amylases, resulting in an increased plasma half-life of the HES solution
An increase in the C2/C6 substitution ratio is inversely correlated with rate of degradation
Highly substituted HES products have a terminal half-life that increases with repetitive dosing as well as with longer infusion durations
Excretion of HES
After initial intravascular hydrolysis by serum amylase, smaller HES molecules (<55 kDa) are excreted unchanged in the urine or are taken up into tissues, namely, cells of the reticuloendothelial system
Cellular uptake of larger HES molecules (>55 Kda) also occurs but with decreased frequency and subsequent slow intracellular degradation ensues
Cellular lysosomes lack the amylase enzymes required for efficient HES hydrolysis, making the half-life of HES in tissues far longer than in plasma
Volume of Distribution of HES
In the presence of an intact, healthy ESL, the volume of distribution of HES solutions is theoretically limited to the intravascular space
The large osmotically active particles in these solutions retain water within the plasma compartment and impede tissue edema
The large MW of HES molecules allows for absorption into the glycocalyx layer of the ESL and further augments resistance to ultrafiltration of fluid across the capillary barrier
Adverse Effects of HES
Most significant
Coagulation disorders
AKI
Increased mortality
Other documented adverse effects
Pruritis
Reticuloendothelial dysfunction
Hepatopathies
Anaphylactoid reactions
Specific patient populations, particularly septic patients, appear to be at higher risk of adverse effects, and administration of artificial colloids to these patients may be associated with increased mortality
Coagulopathy Due to HES
Mechanism of coagulopathy not completely known
Thought to be mediated by direct effects of colloid molecules on the coagulation system and not exclusively via hemodilution
Effects are dose-dependent
Administration of HES has been shown to lead to platelet dysfunction, reduced von Willebrand factor (vWF) and factor VIII activity, and an acquired fibrinogen deficiency or dysfunction
Both gelatin and dextran solutions have demonstrated interference with both primary and secondary hemostasis
Clinical data has also revealed reduced fibrinogen concentrations, impaired fibrin crosslinking, and compromised clot stability associated with the administration of both dextran and HES solutions
Hemodilution with HES results in a weaker clot, less stable fibrin network, and less firm platelet aggregation as compared to crystalloid or albumin hemodilution
Viscoelastic coagulation studies in human and veterinary patients have demonstrated patterns consistent with hypercoagulability following both in vivo and in vitro HES hemodilution
Recent data evaluating tetrastarches have shown reduced, but not absent HES-associated coagulation abnormalities and clinical bleeding tendencies
Pathophysiology of HES-Induced Platelet Dysfunction
Hydroxyethyl starch molecules induce cellular abnormalities that result in decreased agonist-induced expression of the glycoprotein (GP) aIIb/B3 receptor on platelet surfaces, culminating in inhibited platelet adhesion and aggregation and prolonged measured platelet closure time (CT)
Evidence that HES molecules may bind to an coat the platelet surface, leading to further inhibition of activation, aggregation, and adhesion to fibriongen
Effects on platelet dysfunction are more pronounced with administration of early generations of HES
Changes in Secondary Hemostasis Due to HES
Acquired von Willebrand syndrome with decreased factor VIII activity is a recognized consequence of administration of all classes of synthetic colloids
Data has shown up to 80% reduction in circulating factor VIII and vWF activity after HES administration within the manufacturer dosing range
Hyperviscosity-Mediated Ischemic Injury Theory of AKI from HES
Largely discounted
Theoretically possible if a hyperoncotic colloid (e.g. 10% HES solutions) is used in a dehydrated patient
Filtration of HES molecules into the tubular fluid generates a hyperviscous urine, resulting in stasis of flow and obstruction of the tubular lumen
Mechanism of injury relies on the creation of hyperonocotic tubular fluid which has debatable clinical relevance in the setting of critical illness
Osmotic Nephrosis from HES Causing AKI
Process of vascuolization and swelling of proximal renal tubular cells after exogenous solute administration within a few hours of exposure
Tubular lesions are reversible structural changes that can be associated with AKI and have been documented with both early and late generation HES administration
Preferential uptake of HES molecules occurs in proximal tubular luminal epithelial cells via pinocytosis and intracellular lysosomal storage can lead to accumulation of intracellular water, cytoplasmic swelling, and altered cellular integrity and function, culminating in tubular damage and the clinical syndrome of AKI
Tissue Accumulation and Pruritis with HES
HES molecules have been shown to accumulate in numerous tissues after infusion including reticuloendothelial cells, dermal cells, nerve cells, and hepatocytes
Tissue uptake of HES is both dose- and time-dependent
HES can accumulate in cytoplasmic vacuoles that can persist for long periods of time even after relatively low-dose infusions of a HES solution for volume replacement
Tissue uptake in small peripheral nerves and skin is implicated in HES-associated pruritis
Reported in 30-60% of HES-treated human patients
Delayed onset (typically 1-6 weeks postexposure)
Refractory to available therapies
Can last up to 24 months
Current data have failed to support previous hypotheses that newer generation lower MW HES solutions will have reduced tissue uptake and expedited renal excretion
A recent meta-analysis demonstrated a 42.3% overall tissue uptake of low MW HES (<200 kDa) compared with a 24.6% tissue uptake of high MW HES
Efficacy of a Resuscitation Fluid
Its ability to restore effective circulating blood volume, organ perfusion, and tissue oxygenation while minimizing necessary fluid loading that could contribute to a positive fluid balance
Volume Sparing Effect of Colloids vs Crystalloids
In several recent studies in critically ill human patients, the volume-sparing effect of colloidal solutions as compared to crystalloids has been margin, with intravascular volume expansion efficacy close to a ratio of 1.2-1.4:1 for crystalloid:colloid
Marginal differences in fluid volumes needed to restore effective circulating volume between crystalloids and colloids diminishes the advantage of a colloid
Past data from healthy humans and experimental animals demonstrated 3-4 times as much crystalloid than colloid infusion are needed to achieve an equivalent increase in intravascular volume
Data show colloidal solutions have a larger than predicted volume of distribution in critically ill patients
What is colloidal resuscitation most efficacious with?
Colloidal resuscitation has been demonstrated to be most efficacious in the treatment of intravascular hypovolemia, with 80-100% of the infused volume remaining in the intravascular space
Administration of a bolus of isooncotic fluids into the circulation of a normovolemic patient has a reduced plasma volume-expanding effect, with upwards of 60% of the infused volume being shifted into the interstitium
This increased volume of distribution is proposed to be mediated by hypervolemia-associated damage to the endothelial barrier
Iatrogenic hypervolemia stimulates the release of ANP which plays a direct role in EG degradation
Importance of Intact Endothelial Surface Layer
The revised Starling model and glycocalyx paradigm of transvascular exchange highlight the importance of an intact ESL in the maintenance of transcapillary fluid exchange and provides new insights into the physiology of microvascular fluid flux
The oncotic pressure differential across the EG layer limits, but does not eliminate or reverse fluid movement from the intravascular space to the interstitium
Fluid resabsorption from the interstitium does not occur in the venous capillary bed, but rather the majority of filtered fluid is returned to the vasculature via lymphatics
A healthy EG is essential to avoid capillary leak and tissue edema formation
Safety of HES
HES solutions are associated with clinically significant adverse effects, the most detrimental being coagulopathies and AKI
Cochrane review assessed all RCT comparing colloids versus crystalloids for fluid resuscitation in critically ill patients
An overall increased risk of AKI, RRT, and kidney failure was found in HES-treated individuals
No significant differences were found between septic and non-septic patients
Specific HES solution formulation (MW, MS, C2/C6) and total HES does did not impact development of AKI across included studies
A second Cochrane review evaluated the effect of HES on kidney function, as compared with any other fluid therapy
A significant increase in the need for RRT was found in the HES-treated individuals
The risk of meeting RIFLE-R (risk) criteria for AKI was higher in patients receiving any fluid other than HES, HES-treated individuals were more susceptible to development of more severe RIFLE outcomes
No differences in need for RRT and RIFLE-F based outcomes were seen between sepsis versus nonsepsis patients in subgroup analysis, between high versus low MW and MS HES solutions, or based on total volume of HES administered
Concluded that all HES products increase the risk of AKI and RRT across patient populations and that a safe volume of any HES solution has yet to be determined
Additional meta-analysis in late 2013
Found incidence of AKI and need for RRT were significantly higher in patients receiving HES
Concluded that in most clinical situations it is likely that these risks outweigh any benefits and that alternative volume replacement therapies should be used in place of HES products
Currently no published data confirming an association between HES administration and AKI in the veterinary population
Outcome Following HES Administration
Minimal clinical evidence that the incorporation of colloidal solutions into fluid resuscitation algorithms affords a survival advantage
Resuscitation with colloidal solutions in lieu of a purely crystalloid driven regimen in the setting of severe illness has not demonstrated a survival advantage
The reported adverse effects associated with HES have been compelling enough to prompt the current Surviving Sepsis Campaign Guidelines to recommend against the use of HES for fluid resuscitation of severe sepsis and septic shock (grade 1B)
Instead suggest the incorporation of HES in the fluid resuscitation plan of patients with severe sepsis and septic shock when substantial amounts of crystalloids are required (grade 2C)
The use of HES solutions has been banned from the use in the European market
Veterinary Implications of HES
The total administered volume of most resuscitation fluids is far less in veterinary patients due to the shorter mean duration of hospitalization as well as lower illness severity and perceived global volume deficits
The HES-associated adverse effects of AKI and mortality have been noted to be dose and time dependent in some trials
HES associated AKI typically becomes evident 5-20 days after ICU admission
The abbreviated hospitalization in veterinary patients may mitigate the risk of HES toxicity
Patients in the CRYSTMAS trial who were treated with HES for only 4 days did not develop a HES-associated AKI
Some subgroup analyses have shown renal impairment within 3 days in high-risk patient populations, which may represent a risk for the more severely ill cohorts
A species-specific, readily available, cost effective natural colloid solution does not currently exist for veterinary patients
With the known morbidity associated with large volume crystalloid administration and positive net fluid balance, there is a drive to provide resuscitation that may limit these effects
Adjunct colloidal support is often required to adequately resuscitate a patient with substantial circulatory failure and achieve normalization of hemodynamic parameters without causing a net positive fluid balance
The CRISTAL and CRYSTMAS trials may be more applicable to veterinary ICU patients as these focused on hypovolemic patients with severe volume deficits requiring rapid correction and short duration HES use
Veterinary patients may not be as susceptible to or at risk for the adverse effects documented in human patients
Judicious, thoughtful use of HES in veterinary patients is merited, with consideration of potential side effects
Adherence to manufacturer recommended daily dosing is advocated with consideration of earlier use of vasopressors and inotrope therapy in the treatment of circulatory failure once initial fluid resuscitation has failed
There is evidence supporting avoidance of use in HES solutions in certain human patient populations such as patients with known renal dysfunction and patients with sepsis, systemic inflammation or suspected severe capillary leak and third spacing
When should you use HES in horses with septic shock?
Only if crystalloids are insufficient
What type of HES should you use in horses with septic shock?
Tetrastarches, pentastarches, or hetastarches
Dose of HES in Horses with Septic Shock
5-10 ml/kg
Type of Administration of HES for Horses in Septic Shock
Bolus
Objective of Administration of HES in Horses with Septic Shock
Early goal-directed resuscitation; volume expansion
Alternatives to HES for Horses in Septic Shock
Crystalloids
Repeated Administration of HES for Horses in Septic Shock
Avoid
When should you administer HES for horses in hemorrhagic shock?
Signs of hypovolemia and impaired tissue oxygenation
What type of HES should you administer in horses with hemorrhagic shock?
Tetrastarches, pentastarches, or hetastarches
Dose of HES for Horses in Hemorrhage Shock
10-15 ml/kg
Type of Administration of HES for Horses in Hemorrhagic Shock
Bolus
Objective for HES Administration in Horses with Hemorrhagic Shcok
Hypotensive resuscitation; volume expansion
Alternatives to HES in Horses with Hemorrhagic Shock
Crystalloids
Blood
Repeated Administration of HES in Horses with Hemorrhagic Shock
Only if needed
When should you administer HES in horses with hypoalbuminemia?
Severe hypoalbuminemia <15 g/L; Moderate hypoalbuminemia 15-20 g/L with needs of intravenous fluid support