OCHEM 2 Lab Final: Aspirin
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
34 Terms
What statements are always true about limiting reactants?
The limiting reactant is completely used up in the reaction
The limiting reactant dictates the amount of product
There will be an excess of other reactants at the end of the reaction
Identify the medical applications of aspirin
Pain killer, fever reducer, anti-inflammatory agent
What is this?
Aspirin
Why do you need to support a vacuum filtration apparatus with a clamp?
The filter funnel makes the apparatus top-heavy
When using vacuum filtration to separate a dissolved solid from an undissolved solid, what techniques should you use to ensure a quantitative separation?
Carefully rinse the flask into the filter funnel with a small amount of water
Wet the filter paper before pouring the mixture into the filter funnel
Wash the solid on the filter paper with a small amount of water
Dry the solid on the filter paper after the separation
Iron(III) chloride can be used to assess the purity of aspirin synthesized from salicylic acid. Iron(III) chloride **** react with aspirin because the reaction requires a *** which is present in ****.
does not, phenolic functional group, salicylic acid but not aspirin
Once the vacuum filtration set-up is complete, start by **** in the filter funnel. Then, *** the mixture ****.
adding a small amount of water, slowly decant, into the middle of the filter paper
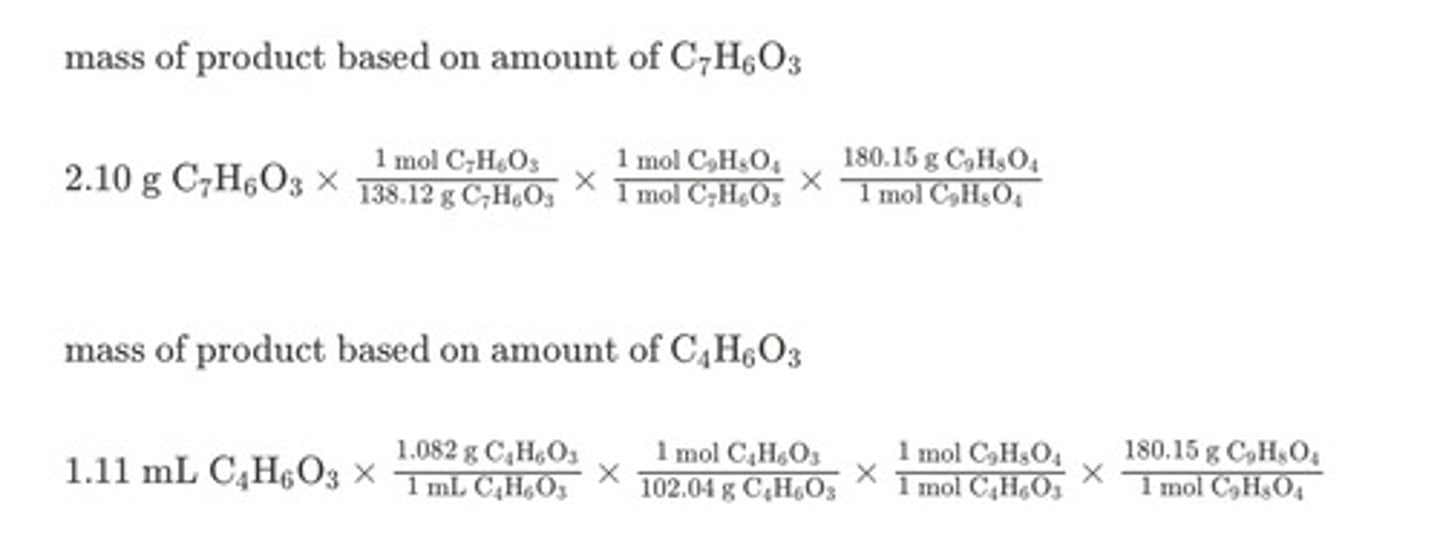
Aspirin can be prepared from salicylic acid (C7H6O3), which has a molar mass of 138.12 g/mol, and acetic anhydride (C4H6O3), which has a molar mass of 102.04 g/mol. The density of acetic anhydride is 1.082 g/mL. C7H6O3 + C4H6O3 ⟶ C9H8O4 + C2H4O2
What is the yield, in grams, of aspirin (C9H8O4), which has a molar mass of 180.15 g/mol, possible when reacting 2.10 g of salicylic acid with 1.11 mL of acetic anhydride?
2.12
Iron Test: Salicylic acid
Dark purple
Phenol
Iron Test: Commercial aspirin
Pale yellow
No Phenol
Explain why acetic acid is unlikely to be a contaminant in your solid aspirin.
Acetic acid will not crystallize due to the water and will therefore not contaminate the solid aspirin. Acetic acid is water soluble. We remove acetic acid while washing the product with water on the filter during the filtration.
Aspirin tablets that have been stored for a long time may have a vinegar-like odor and give a purple colour with FeCl3. What reaction would cause this to happen?
A purple colour with FeCl3 means that salicylic acid is present. A vinegar-like odor means that acetic acid is present. These two facts mean that the product started degrading to salicylic acid and acetic acid, which means the aspirin synthesis is a reversible reaction.
In preparation of aspirin, if the water used for washing crystals is not cold, how would this affect the melting range of final product? Explain!
Using warm or room temperature water for washing the crystals in the preparation of aspirin can negatively impact the melting range of the final product. It can result in lower yields, impurity retention, and the formation of larger or less-defined crystals. Cold water is preferred because it promotes rapid cooling, crystal formation, and effective removal of impurities, ultimately leading to a more precise and narrow melting range
In preparation of aspirin, if crystals of the final product is not dried, how would this affect the melting range of final product? Explain!
Failure to properly dry the crystals of the final product in the synthesis of aspirin can introduce moisture, impurities, and solvent effects, all of which can influence the melting range. To obtain accurate and meaningful melting point data, it is essential to thoroughly dry the crystals to remove moisture and ensure the purity and integrity of the final product
In preparation of aspirin, if crystals of the final product is not dried, how would this affect the calculated percent yield? Explain!
The failure to properly dry the crystals of the final product can lead to an overestimation of the actual yield and an inflated percentage yield. Thorough drying is essential to remove moisture, prevent the inclusion of impurities, and obtain accurate measurements, thereby ensuring an accurate calculation of the percentage yield. By properly drying the crystals before measuring the actual yield, the weight obtained will reflect the true weight of the acetylsalicylic acid product, without the contribution of moisture or residual solvents. Consequently, the calculated percentage yield will be more accurate and reflective of the efficiency of the reaction and the amount of desired product obtained.
In preparation of aspirin, if the mixture of crystalline product and ethanoic FeCl3 produces a colored solution, how would this affect the purity of product? Explain!
If the mixture of the crystalline product and ethanoic FeCl3 produces a colored solution, it suggests the presence of impurities in the product. The color development indicates the potential presence of related compounds, byproducts, or unreacted starting materials. A colored solution affects the purity assessment by indicating a lower purity of the product. Further analysis with appropriate techniques is recommended to gain a more comprehensive understanding of the impurities and their impact on the overall purity of the synthesized acetylsalicylic acid.
In preparation of aspirin, if the mixture of crystalline product and ethanoic FeCl3 produces a colored solution, how would this affect the melting range of final product? Explain!
if the mixture of the crystalline product and ethanoic FeCl3 produces a colored solution, it suggests the presence of impurities in the product. The impurities can influence the melting range by introducing additional melting peaks, broadening the range, or causing a shift in the melting point. Additional purification and characterization steps are necessary to ensure an accurate determination of the melting range and assess the purity of the synthesized acetylsalicylic acid.
What is the purpose of adding sulfuric acid?
It's a good catalyst to speed up the reaction.
what is the purpose of testing with the FeCl3?
To check if any free OH groups are present, to see if the Aspirin has been purified properly or as close to the commercial aspirin.
Which functional group is present in the product?
The functional groups that are in aspirin are carboxylic acid and ester.
Discuss significance of FECl3 test results
Iron (III) Chloride reacts with phenol groups. If there is an -OH group, it will react. Therefore, since salicylic acid has an -OH group, the FeCl3 will turn the solution purple.
Morphine is an analgesic available at the drug store. Using acetic anhydride and morphine, one can easily synthesize a recreational drug. Which one is it? Hints: Search the structure of morphine. And then search the structures of the the options presented below.
Heroin
In today's experiment, which of the following is the crystallization solvent?
Water
One of the chemicals that you will be dealing today reacts vigorously with small amount of water and creates splash. A similar accident happened at UTEP in the past, and someone lost his one eye due to that. After that accident, UTEP changed the policy of eye protection and now safety glasses are no longer allowed in the lab. Which of the following compounds we are talking here?
Acetic anhydride
What are the byproducts of aspirin synthesis?
Acetic acid
Aspirin synthesis can be classified as which of the following types of reactions?
Esterification
If you increase the amount of acetic anhydride the yield will be improved.
False
Today, you used acetic anhydride, which is an acetylating agent. Which of the following agents can also be used as an acetylating agent? (Negative credits will be applied to incorrect answers. Select 2 correct answers to get full credits.)
Acetyl chloride
Acetyl-coenzyme A
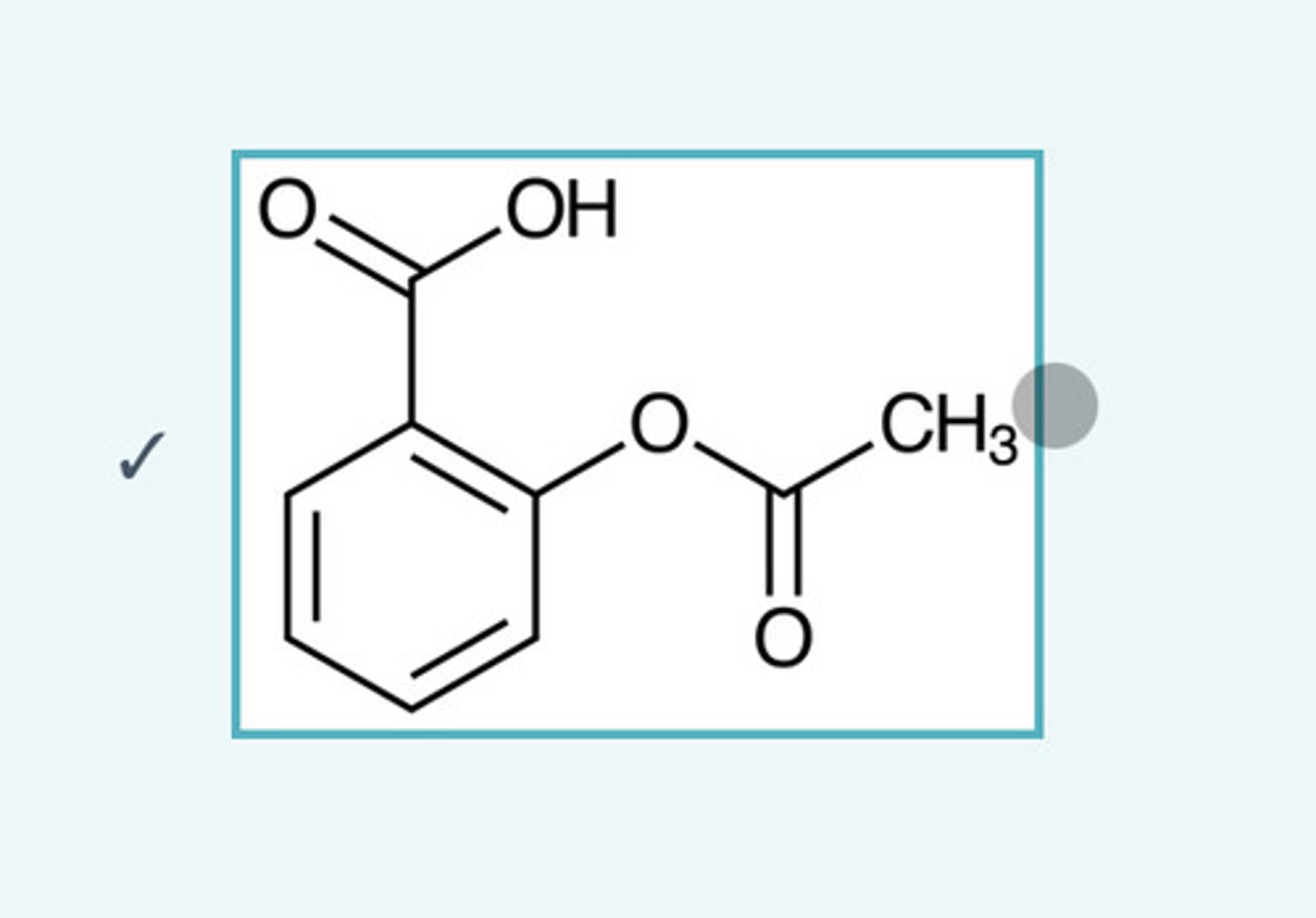
Fill the box with the possible product of the reaction:
Product 3
Which of the following is the first step of the reaction mechanism for aspirin synthesis?
Answer 3
Let's assume that you were given 16.0g salicylic acid, 25.0mL of acetic anhydride and 10 drops of 85% sulfuric acid. If you obtained 80% reaction yield, then how much aspirin you have synthesized?
16.7g
The following compounds are what type of isomers?
Structural Isomer
What is the purpose of adding 15 mL of room temperature water to the heated reaction flask?
To quench/decompose the excess acetic anhydride
What is the limiting reagent in the synthesis of aspirin?
Acetic anhydride