Contraception
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
30 Terms
How does oestrogen affect the menstrual cycle at the hypothalamus and pituitary?
Oestrogen exerts negative feedback on GnRH, LH and FSH during most of the cycle, preventing excessive follicular recruitment. Around mid-cycle, sustained high oestrogen triggers a positive feedback loop, producing the LH surge that causes ovulation. In contraception, oestrogen maintains a constant high level, eliminating the LH surge and preventing ovulation.
How does oestrogen affect the endometrium?
Oestrogen stimulates proliferation of the endometrial lining during the follicular phase, increasing growth of glands and stroma. In combined hormonal contraception, continuous low-dose oestrogen stabilises the endometrium to prevent breakthrough bleeding, but does not allow the natural proliferative-to-secretory transition.
How does oestrogen affect the cervical environment?
Oestrogen produces thin, watery, stretchable cervical mucus (Spinnbarkeit). This facilitates sperm penetration during the fertile window. In contraception containing oestrogen, the progestogen component usually dominates mucus characteristics, so mucus tends to remain thick despite the presence of synthetic oestrogen.
How does progesterone affect the hypothalamus and pituitary?
Progesterone suppresses GnRH pulsatility, which reduces LH and prevents the LH surge required for ovulation. This suppression is the primary mechanism of progestogen-only contraceptives, though suppression may not be complete in all users.
How does progesterone affect the endometrium?
Progesterone converts a proliferative endometrium into a secretory one. In contraception, continuous progestogen causes endometrial glandular atrophy and thinning, making implantation unlikely. This is why progestogen-only methods often cause lighter periods or amenorrhoea.
How does progesterone affect cervical mucus?
Progesterone produces thick, viscous mucus that forms a barrier to sperm entry. This is one of the most reliable contraceptive actions of progestogen-only pills, implants and IUS systems.
How does progesterone affect the fallopian tubes?
Progesterone slows tubal ciliary beating and muscular contractions. This alteration reduces gamete and embryo transport. In contraceptive doses, this contributes to reduced fertilisation probability.
Combined Oral Contraception
COC gives continuous synthetic oestrogen + progestogen, creating a pseudo-pregnancy hormonal state. This suppresses the hypothalamic–pituitary–ovarian (HPO) axis, so ovulation does not occur. Ovulation suppression is the primary mechanism.
Step-by-step
Hormones taken daily
Tablet contains ethinylestradiol (oestrogen) + a progestogen (e.g. levonorgestrel, desogestrel).
Blood levels are steady and higher than normal cycle levels.
Negative feedback on the HPO axis
High oestrogen + progestogen inhibit GnRH from the hypothalamus.
This reduces FSH and LH release from the pituitary.
Ovulation is prevented
↓ FSH → follicles do not mature properly.
↓ LH → no LH surge, so no ovulation.
Cervical mucus thickens (progestogen effect)
Mucus becomes thick and hostile to sperm, reducing sperm entry into the uterus.
Endometrium becomes thin and inactive
The uterine lining is less receptive, making implantation unlikely.
Progesterone is only “pro-implantation” when:
It rises after ovulation
On a lining already thickened by oestrogen
In COC, progesterone is early, constant, and synthetic, so it has the opposite effect.
Why is ovulation not always absolutely suppressed in some CHC users?
Pharmacokinetic variation affects hormone levels; metabolism differs between individuals; missed pills or absorption issues can temporarily reduce suppression. Some degree of follicular development may still occur but usually without ovulation.
What are major benefits of combined hormonal contraception?
It is reliable, rapidly reversible, unrelated to intercourse, and under the woman’s control. It reduces ovarian and endometrial cancer risk by half, helps endometriosis, reduces dysmenorrhoea, menorrhagia and PMS, and allows cycle control including continuous use to stop periods.
What are risks of combined hormonal contraception?
Most COC risks come from oestrogen effects on clotting and liver metabolism and progestogen effects on vascular tone and metabolism. All listed risks are rare, but certain risk factors increase likelihood.
1. Cardiovascular risks
Arterial (mainly progestogen-related, risk-modified)
Includes stroke and myocardial infarction
Risk increases with:
Smoking (especially >35 years)
Hypertension
Mechanism:
Progestogens can affect lipids and vascular tone
Smoking + oestrogen/progestogen → endothelial damage + thrombosis risk
Venous (mainly oestrogen-related)
Includes VTE:
DVT
Pulmonary embolism
Mechanism:
Oestrogen increases clotting factors (II, VII, IX, X) and reduces anticoagulants
Higher risk in:
Inherited clotting disorders
Obesity
Immobility
Migraine with aura (important contraindication)
2. Neoplastic risks
Breast cancer:
Slight increase while using COC
Risk returns to baseline after stopping
Cervical cancer:
Slight increase with long-term use
Liver tumours:
Rare (hepatic adenomas), oestrogen-related
Protective effects (important exam point):
↓ Ovarian cancer
↓ Endometrial cancer
3. Gastrointestinal / metabolic
Insulin resistance / carbohydrate metabolism
Oestrogen can reduce insulin sensitivity
Weight gain
Evidence weak; often due to fluid retention rather than fat gain
4. Hepatic risks
Liver metabolises steroid hormones → increased hepatic workload
Can worsen:
Congenital non-haemolytic jaundice
Cholestasis
↑ Risk of gallstones
Oestrogen increases cholesterol in bile
5. Dermatological
Chloasma (melasma)
Oestrogen increases melanocyte activity
Acne
Depends on progestogen type (some androgenic, some anti-androgenic)
Erythema multiforme
Rare immune-mediated reaction
6. Psychological
Mood swings
Depression
Changes in libido
Mechanism:
Steroid hormones interact with neurotransmitters (serotonin, GABA)
How do liver enzyme-inducing drugs affect CHC?
Drugs such as carbamazepine, phenytoin, rifampicin, topiramate and certain antiretrovirals increase hepatic metabolism of contraceptive hormones, lowering serum levels and reducing efficacy. Additional barrier methods are required.
What are the pill rules for CHC?
Start on day 1 of menstruation. Take 21 days of pills followed by a 7-day pill-free interval, restarting on day 8. Late or missed pills in the first week require condoms. Missing pills in the last 7 days means skipping the pill-free interval. Annual BP and BMI checks are required
How does the vaginal ring work?
The ring delivers the same hormones as CHC but via the vaginal route. It stays in for 21 days, is removed for 7 days, and then replaced. It avoids daily pill taking but still relies on correct insertion and timely replacement.
Progestogen-only contraception?
Progestogen creates a hostile reproductive environment—mainly by thickening cervical mucus. Ovulation suppression happens variably, depending on dose and delivery method.
What progestogen does in the body
1. Thickens cervical mucus (main mechanism for all methods)
• Mucus becomes thick, viscous, and impermeable
• Sperm cannot penetrate the cervix
• This effect happens even at low hormone levels
2. Thins the endometrium
• Progestogen suppresses endometrial proliferation
• Lining becomes thin, inactive, and unreceptive
• Implantation is unlikely
3. Suppresses ovulation (method-dependent)
• High, continuous progestogen → suppresses LH surge
• Reliability varies:
• Desogestrel POP, implants, injections → ovulation usually suppressed
• Traditional POPs → ovulation often still occurs
4. Reduces tubal motility
• Slows ciliary action and smooth muscle contraction
• Reduces sperm–egg transport efficiency
2. Progestogen-only methods
A. Long-acting reversible contraception (most reliable)
• Implants (e.g. etonogestrel)
• Hormone-releasing IUS (levonorgestrel)
• Injectables (e.g. medroxyprogesterone acetate)
Mechanism:
• Cervical mucus thickening
• Endometrial thinning
• Consistent ovulation suppression
B. User-dependent methods (POPs)
• Daily tablets
• Mechanism depends on type:
• Desogestrel POP → usually suppresses ovulation
• Older POPs → mainly rely on cervical mucus
• Timing is critical (missed pills reduce efficacy)
3. Why there is no oestrogen
• Avoids oestrogen-related risks:
• VTE
• Hypertension
• Migraine with aura
So it’s safe to use in:
• Breastfeeding
• Smokers >35
• Many cardiovascular risk groups
Copper IUDs
Copper IUCDs create a toxic, inflammatory intrauterine environment that is hostile to sperm and implantation. They work without hormones.
Step-by-step
Copper ion release
The device continuously releases Cu²⁺ ions into the uterine cavity.
Direct spermicidal effect (primary mechanism)
Copper ions:
Are toxic to sperm
Reduce sperm motility and viability
Increase reactive oxygen species
Fertilisation is usually prevented before sperm reach the egg.
Sterile inflammatory reaction
Copper acts as a foreign body → local inflammatory response
↑ Leukocytes, macrophages, and prostaglandins
This environment is hostile to:
Sperm
Eggs
Early embryos
Endometrial effects
Prostaglandins + inflammation → endometrium unsuitable for implantation
Also causes:
↑ uterine contractility
↓ implantation probability
Mechanical contribution
Physical presence of the device adds a minor mechanical barrier, but this is not the main effect
Important clarifications (exam-relevant)
Copper IUCDs do not disrupt an established pregnancy
Main action is pre-fertilisation
Can be used as emergency contraception (up to 5 days post-intercourse)
No systemic hormonal effects
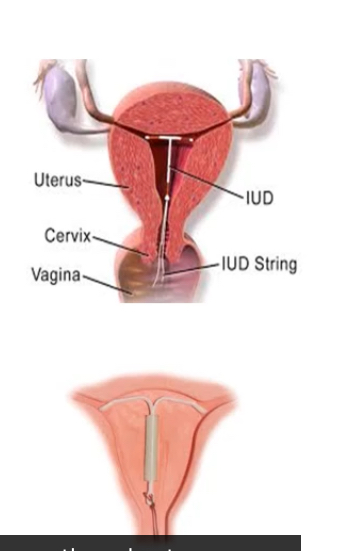
What are benefits of IUCDs?
They are extremely reliable, immediately effective, non-user dependent, long-acting, coitus-independent, rapidly reversible and free from major systemic risks
What are disadvantages of IUCDs?
They require trained insertion, may cause pain during fitting, may increase menstrual pain or bleeding (copper types), do not protect against STIs, and threads may be felt by partners.
What are risks of IUCDs?
Rare expulsion, rare uterine perforation, miscarriage risk if pregnancy occurs with IUCD in situ, and a small risk of ectopic pregnancy if pregnancy occurs.

What are absolute contraindications for IUCDs?
Current pelvic inflammatory disease, pregnancy, unexplained vaginal bleeding, and uterine cavity abnormalities.
Condoms
They create a physical barrier preventing semen from entering the vagina. They also provide protection against STIs by preventing direct mucosal contact and limiting fluid exchange.
What are advantages and disadvantages of male condoms?
Advantages include user control, STI protection and availability. Disadvantages include need for correct timing, potential latex allergy, potential erection-related issues, and higher failure rates with imperfect use.
What are advantages and disadvantages of female condoms?
Advantages include user control, STI protection, and usability independent of male erection. Disadvantages include cost, obtrusiveness, noise during intercourse and uncertain real-world failure rates.
Diaphragms and Caps work?
They are barrier methods that physically block the cervix, stopping sperm from entering the uterus. They rely on correct placement + spermicide.
Step-by-step
Insertion before sex
The diaphragm or cap is inserted into the vagina before intercourse.
It is positioned to cover the cervix completely.
Barrier effect
The device forms a physical barrier over the cervical os.
Sperm cannot pass from the vagina into the uterus.
Spermicide use (essential)
Spermicide is applied to:
Kill sperm
Immobilise sperm that reach the device
Barrier alone is not sufficient.
After intercourse
Device must be left in situ for at least 6 hours.
This ensures any remaining sperm are inactivated.
Removing it earlier increases pregnancy risk.
2 types:
Diaphragm caps
Made of latex
Fit across the vagina, covering the cervix
Come in different sizes (needs fitting)
Cervical (suction) caps
Made of plastic
Smaller, fits directly onto the cervix by suction
Different sizes available
Key limitations (exam-relevant)
User-dependent → higher failure rate
Must be fitted correctly
No STI protection
Less effective in parous women (especially cervical caps)
What are advantages and disadvantages of diaphragms and caps?
Advantages include user control, advance insertion and perceived naturalness. Disadvantages include training requirements, higher failure rates, messy spermicide use and increased UTI or Candida risk.
How does fertility awareness–based contraception work?
It predicts the fertile window using cycle length, cervical mucus, basal body temperature and ovulation signs. Couples avoid unprotected sex during the fertile period or target intercourse during that window when trying to conceive.
Why is fertility awareness method effectiveness highly user dependent?
It requires accurate daily observation, partner cooperation, teaching from a trained practitioner, and consistent abstinence or alternative contraception during the fertile window. Any deviation reduces reliability.
Emergency Contraception
Emergency contraception works by preventing or delaying ovulation or by preventing fertilisation/implantation, depending on the method and timing. It does not terminate an established pregnancy.
1. Postcoital (emergency) pills
A. Levonorgestrel (LNG)
Time window: up to 72 hours after unprotected sexual intercourse (UPSI)
Main mechanism:
Delays or inhibits ovulation if taken before the LH surge
Effectiveness:
Prevents ~7 out of 8 expected pregnancies
Key limitation:
Ineffective if ovulation has already occurred
B. Ulipristal acetate (ellaOne)
Time window: up to 120 hours (5 days) after UPSI
Main mechanism:
Potent ovulation delay, even close to the LH surge
Effectiveness:
More effective than levonorgestrel near ovulation
Pills mainly act in the first half of the cycle → “beware” if ovulation has already occurred.
2. Copper IUCD (most effective method)
Time window:
Up to 5 days after ovulation, or
Up to 5 days after UPSI at any time in the cycle
Mechanism depends on cycle phase:
Before ovulation: copper is toxic to sperm → prevents fertilisation
After ovulation: induces inflammatory endometrial response → prevents implantation
Failure rate: extremely low (most effective emergency method)
Provides ongoing contraception once inserted
Exam-critical distinctions
Emergency contraception ≠ abortion
Pills → mainly ovulation delay
Copper IUCD → works before and after ovulation
What is the difference between perfect use and typical use when measuring contraceptive efficacy?
Perfect use refers to method use exactly as prescribed without errors. Typical use includes real-world behaviours such as missed pills or incorrect barrier use. Typical use failure rates are often significantly higher, especially for user-dependent methods.
Why are long-acting reversible contraceptives (LARCs) considered most effective in real-world settings?
They eliminate daily or situational user involvement, removing adherence errors. Implants, IUS and IUCDs show minimal difference between perfect use and typical use, making them the most reliable options.