Unit 1: Biochemistry - Grade 12 Exam Review
1/99
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
100 Terms
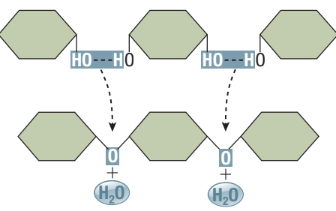
Dehydration Synthesis Reaction (Condensation)
Assembly of macromolecules
Removal of an -OH from one reactant and -H from another reactant
The -OH and -H form H2O, while the two reactants join together forming a covalent bond
Type of Anabolic Reaction: Used to assemble small molecules together into larger ones
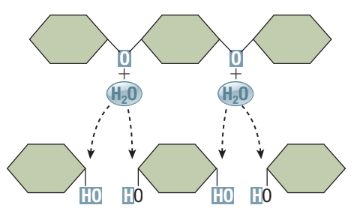
Hydrolysis Reaction
Reverse of dehydration reactions
Disassembly of macromolecules
Water is a reactant to split a large molecule into smaller subunits
A covalent bond in the reactant molecule is broken and the -H and -OH from the water are attached, forming two products
Catabolic Reaction
Macromolecules broken down into subunits (eg. digestion)
Eg. Hydrolysis
Properties of Water
Universal Solvent
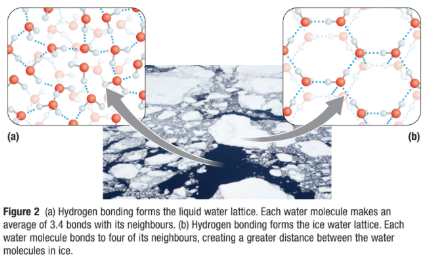
Hydrogen bonds form between water molecules in both liquid and ice, forming a water lattice.
Liquid Water
Hydrogen bonds that hold the lattice together, constantly breaking and reforming in new configurations.
This gives liquid water its ability to float.
Ice
Water lattice is a rigid crystalline structure
Each water molecule in ice forms four hydrogen bonds with neighboring water molecules
This spaces water molecules farther apart that in liquid, so ice is less dense
Specific Heat Capacity
Specific heat: Amount of thermal energy required to increase the temperature of a given quantity of water by a degree.
As heat is added to water, most is absorbed by the process of breaking the H-bonds in the water lattice, which increases water temperature slowly
Water stays liquid until 100 degree C
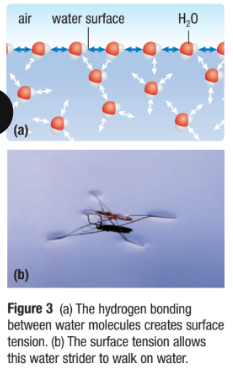
Cohesion (Water Sticks to Water)
A property of water where H-bond lattice results in water molecules staying close together
This creates surface tension: how difficult it is to stretch or break the surface of a liquid
This allows small insects to walk on water
Adhesion (Water Sticks to Other Stuff)
Property where water molecules can form H-bonds with other polar molecules
Eg. Water sticking to your skin when you get out of the shower
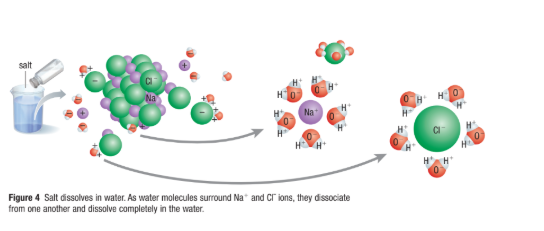
Aqueous Solutions
Water molecules are small and very polar
They surround other polar and charged molecules and ions
This hydration shell, reduces attraction between these other molecules and promote their separation (breaks the ion apart)
This separation allow the substance to dissolve in the solution
Hydrophilic Molecules
Polar molecules or ions that are strongly attracted to and very soluble in water
Hydrophobic Molecules
Non-polar molecules that are not strongly attracted to and soluble in water
Three Types of Carbohydrates
Monosaccharides, Disaccharides and Polysaccharides
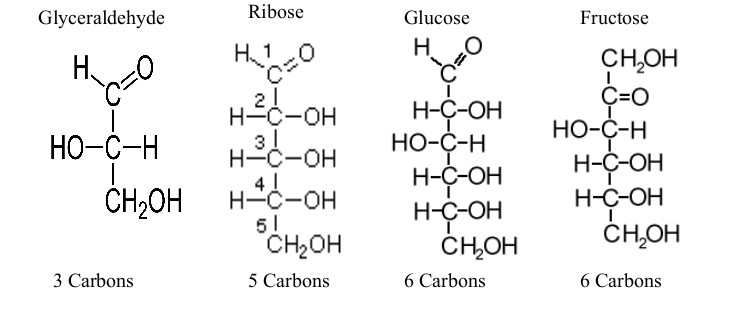
Monosaccharides
“Mono” is single, “Saccharide” is sugar
Simplest sugar
Have ratio of C:H:O = 1:2:1
Distinguished from one another by:
Carbonyl group: either aldehyde or ketone
Length of Carbon chain
What happens when Carbohydrates dissolve in water?
Monosaccharides with five or more carbons are linear in dry state, but form rings when dissolved in water
Example: When glucose dissolves in water, the -OH group at carbon 5 reacts with the aldehyde group at carbon 1 to form a closed ring
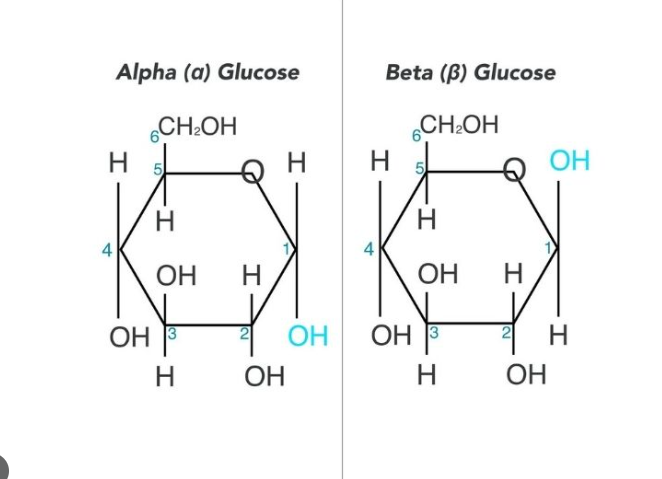
Beta-Glucose
50% chance the -OH group at Carbon 1 will end up above the plane of the ring
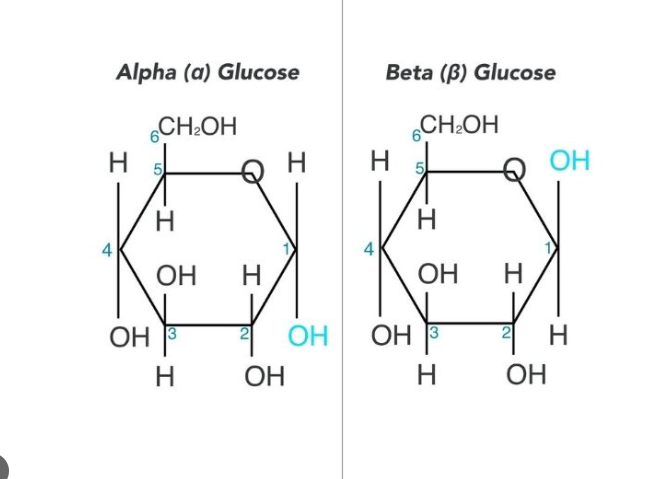
Alpha-Glucose
50% chance the -OH group at Carbon 1 will end up below the plane of the ring
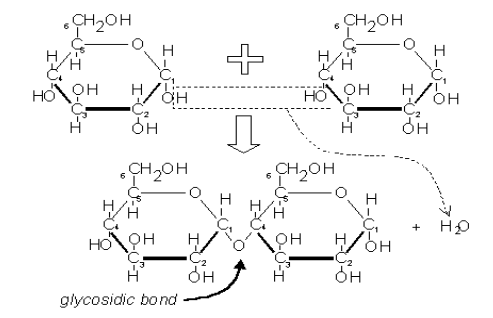
Disaccharides
Sugars containing two (disaccharides) simple sugars
Linked together by a 1-4 glycosidic linkage: A covalent bond between 2 monosaccharides by a condensation reaction (dehydration synthesis)
The hydroxyl group of carbon 1 of the glucose molecule links with the hydroxyl group of carbon 4 of the adjacent molecule:
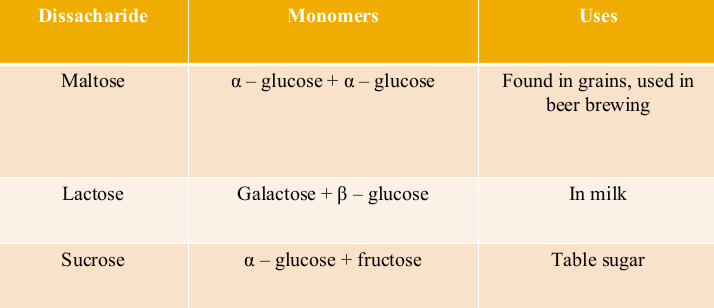
Disaccharide Chart
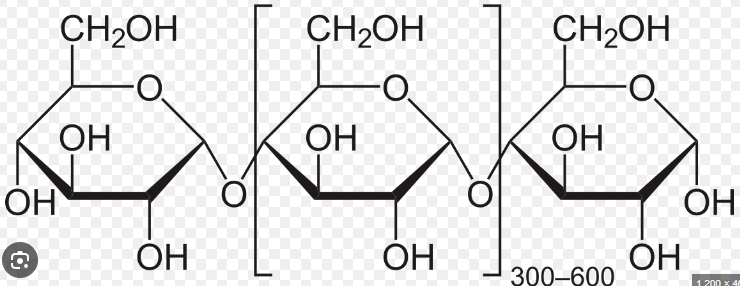
Polysaccharides
Formed by linking monosaccharides (several 100 to several 1000) by glycosidic linkages
Can be straight chained, or branched
Very polar due to many hydroxyl groups
Hydrophilic but will not dissolve due to large size
Have two important biological functions:
Energy storage (starch and glycogen)
Structural support (cellulose and chitin)
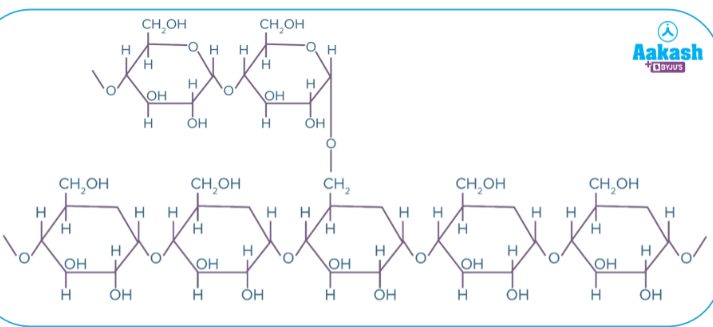
Starch
Main form of energy storage in plants
Plants produce starch by linking excess glucose molecules together
Two types: Amylose & Amylopectin
Amylose
No branches, all 𝛂-1, 4-glycosidic linkages
Amylopectin
Branched, 𝛂-1, 4-glycosidic linkages for main chain and 𝛂-1, 6-glycosidic linkages for branches
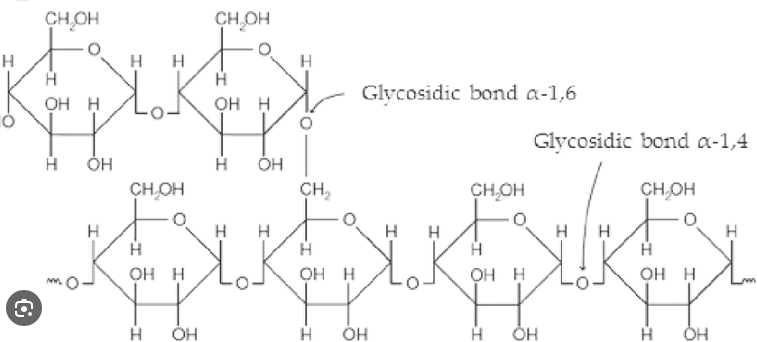
Glycogen
Storage polymer in animals (muscle and liver)
Glycogen stores small; depleted in a day if not replenished
Highly branches: (𝛂 1-4) linked glucose main chain with (𝛂1-6) linked branches
More branching and more compact than amylopectin (starch)
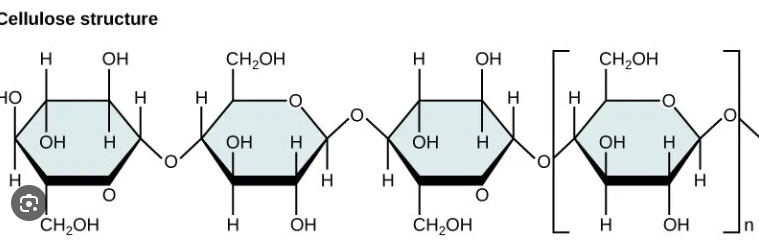
Cellulose
Major component of cell walls
Straight chain polymer of beta-glucose held together by ꞵ-1-4 glycosidic linkages, where every other glucose molecule is inverted
Humans cannot digest cellulose because they lack an enzyme to hydrolyze ꞵ-1-4 linkage (roughage)
Provides rich supply of energy for organisms who can break it down
Lipids
Lipids are nonpolar biological molecules that provide long term energy storage, insulation, cushioning of internal organs and are the main component of the cell membrane
Lipids are the main structure of some hormones
All lipids are hydrophobic (do not dissolve in water)
Five Main Types of Lipids
Fatty Acids
Fats
Phospholipids
Steroids
Waxes
Fatty Acid
Consist of a long chain of carbon and hydrogen atoms with a terminal carboxyl functional group
Carboxyl gives its acidic properties
The longer the chain the more hydrophobic it becomes
What are the two types of Fatty Acids?
Saturated
Unsaturated
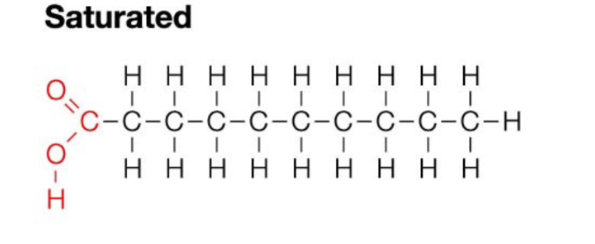
Saturated Fats
Contain the maximum number of hydrogen atoms per carbon atom
No double bonds - a straight chain
Unsaturated Fats
Contain a carbon double bond formed by removal of H from carbon skeleton - chain with a bend in it
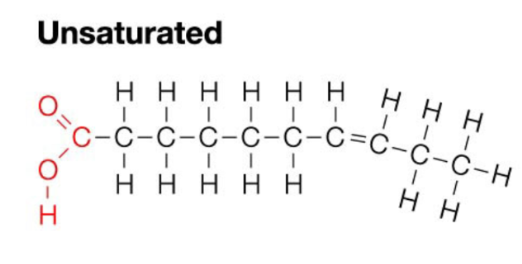
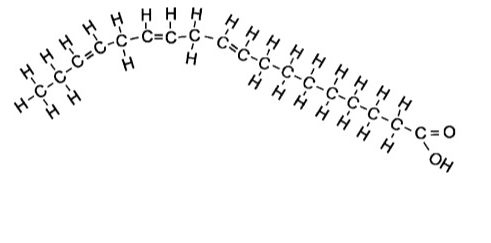
Polyunsaturated
Contains more than one carbon double bond
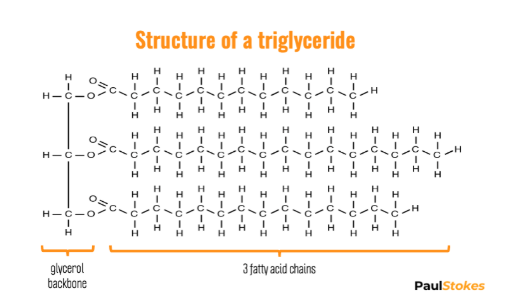
Fats
Energy storage molecule - more than 2x energy than carbohydrates
The most common fat is the triglyceride which contain 3 fatty acid chains attached to a glycerol backbone.
They are linked with a dehydration synthesis reaction and are held together with an ester linkage.
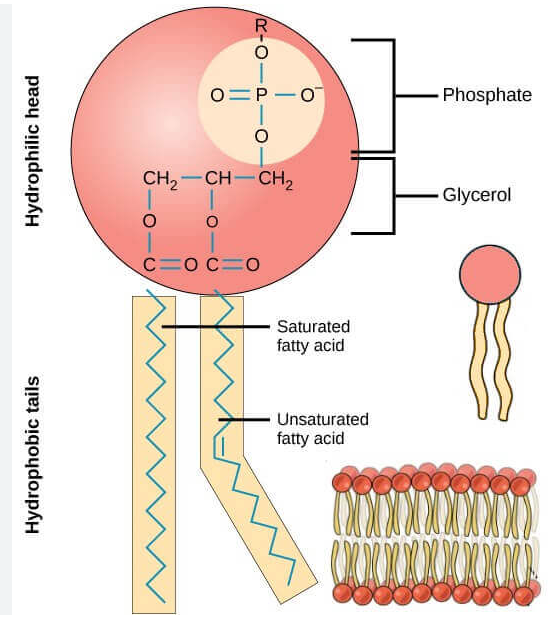
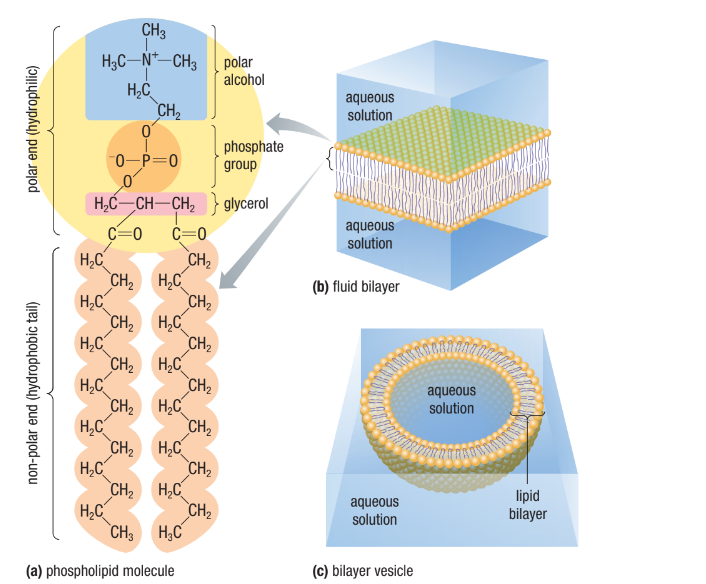
Phospholipids
The main component of cell membrane
Composed of two main parts, a phosphate head and two fatty acid tails
Amphipathic: The phosphate head is polar and hydrophilic while the fatty acid chains are nonpolar and hydrophobic (similar to soap)
Ester and Phosphate ester linkages to a glycerol
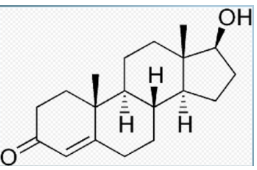
Steroids
Consist of four linked carbon rings
Different steroids have different functional groups attached to the rings
Steroids rings are hydrophobic
Group of steroids Sterols, have a single polar -OH group at one end
Gives molecule dual solubility properties
Includes Cholesterol(component of the cell membrane), and sex hormones
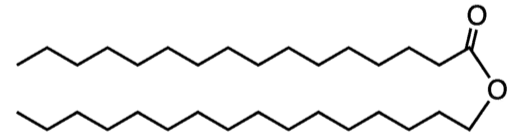
Waxes
Waxes are composed of long-chained fatty acids that are attached to an alcohol or a carbon ring
Waxes are hydrophobic, non-polar and are firm yet pliable
EX. Cutin: Water resistant coating on plants, bird feathers, and beeswax
Proteins
Make up 50% of the dry mass of most cells
Structural value: Collagen (tendons, bones) and keratin(hair, nails)
Enzymes that are used as catalysts in chemical reactions
Transport materials throughout the body like oxygen and carbon dioxide
Produce antibodies that destroy foreign bacteria and viruses
Form structures that allow transport across the membrane
Structure
Proteins are large polymer units made up of amino acids monomers
The overall shape of a protein is determined by the amino acids that it is composed from
The structure of a protein is important in determining its overall function. It has to be the EXACT fit because if the shape changes, then the protein may not be able to perform its function properly
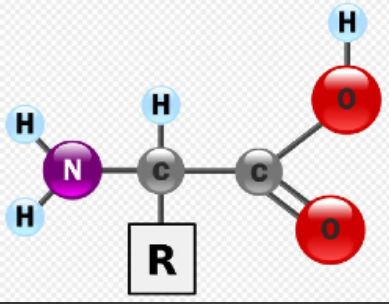
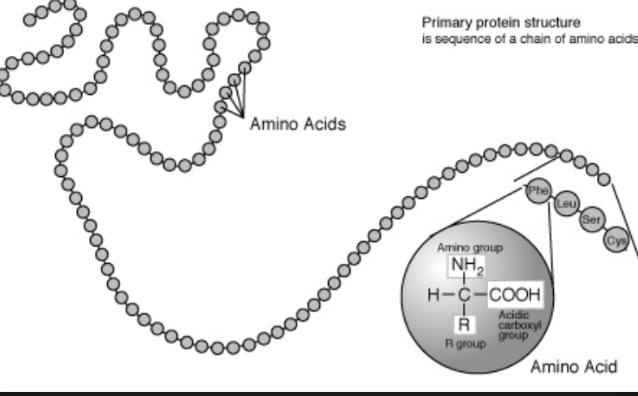
Structure of Amino Acids
Consist of a central carbon bonded to four different covalent partners
Hydrogen atom
Carboxyl
Amino group
R group
There are 20 different R groups which results in 20 different amino acids
R groups give amino acids their properties (polar, non-polar, acidic, etc)
8 amino acids are considered essential - only obtained through diet
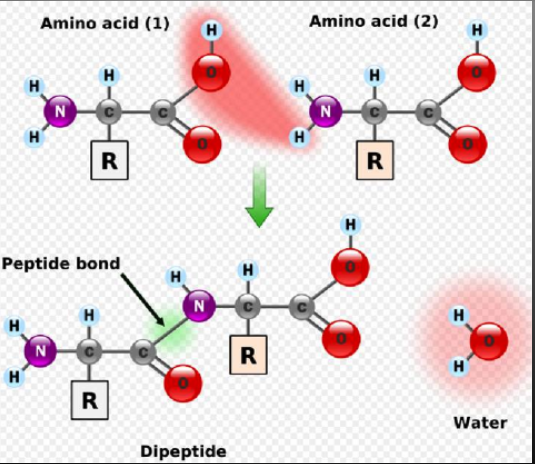
Amino Acid Linkages
Amino acids are linked together through condensation (dehydration synthesis) reactions
The carboxyl group of one amino acid bonds to the amino group of a second amino acid to form a peptide bond.
When two amino acids bond, the resulting molecule is called a diepeptide.
The chain of more than 50 aa’s(amino acids) is called polypeptide
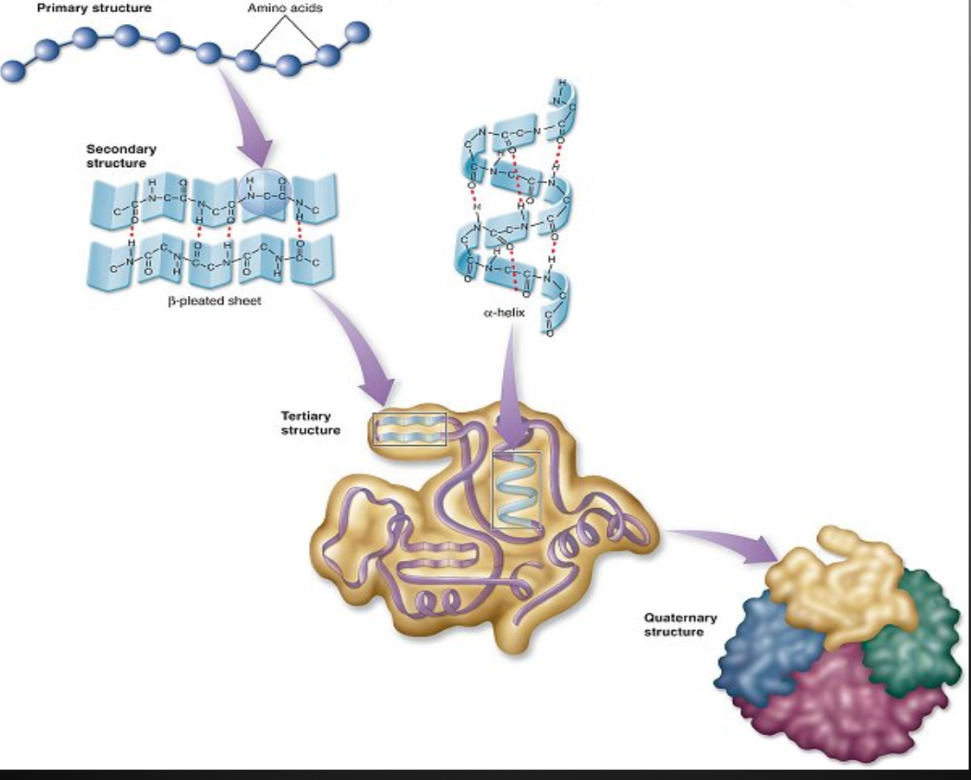
Primary Structure
Unique linear sequence of amino acids in a polypeptide chain
Changing a single amino acid will alter the overall structure of the protein
Unlimited combos of primary structure, specific to each protein (20 combos for each spot of the chain)
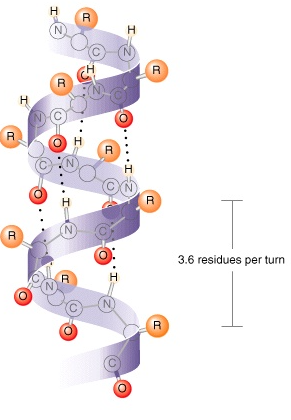
Secondary Structure
Results from hydrogen bonding between carboxyl group of one amino acid and the amino group of a neighboring amino acid
𝛂-helix
Coil structure held together by h-bonds between every fourth amino acids
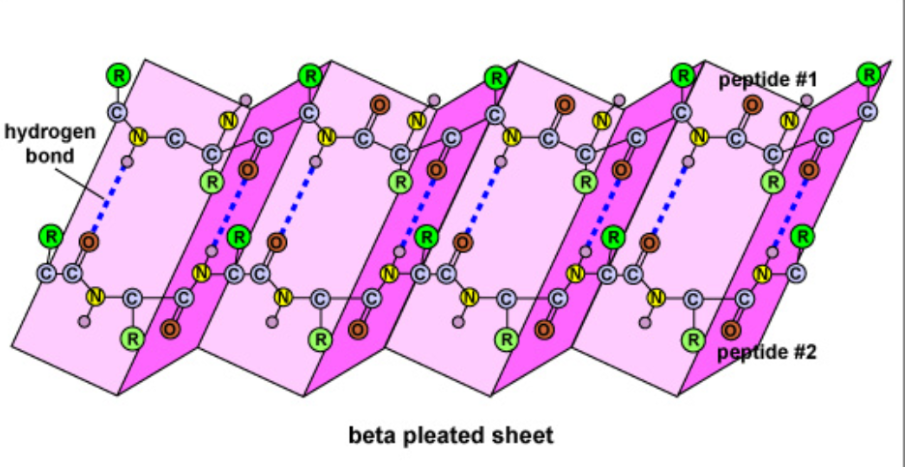
ꞵ-pleated sheet
Two separate polypeptide strands that run parallel to each other interact due to H bonds, an accordion shape appears
Ex. Strength of silk
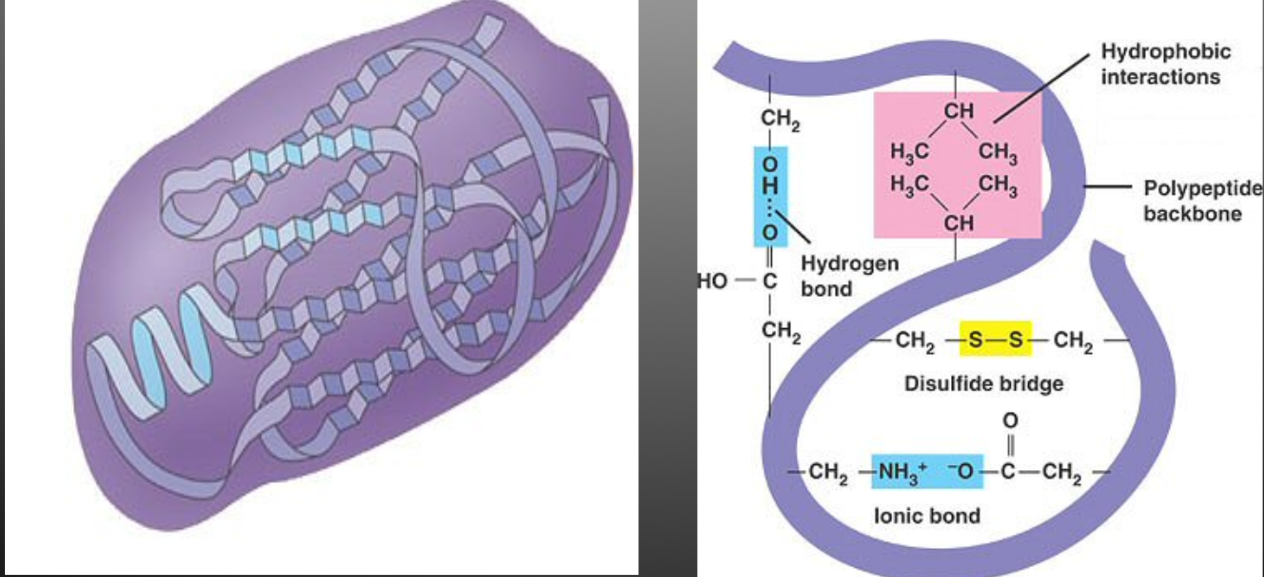
Tertiary Structure
The polypeptide chain continues to bend, fold and contort itself as a result of the interaction between the “R” groups.
Polar, non-polar and ionic “R” groups interact to form hydrogen, covalent, and ionic bonds
Forms a large globular arrangement
EG. Amino acid cysteine contains a sulfur atoms that will form a disulphide bridge with another cysteine atom
Quaternary Structure
Some proteins consist of two or more polypeptide chains combined into one functional macromolecule
Same types of bonds/interactions as tertiary structure
The final structure of a protein (confirmation) is critical as its orientation and shape is directly related to its function. Many diseases and disorders are a result of an improperly functioning protein.
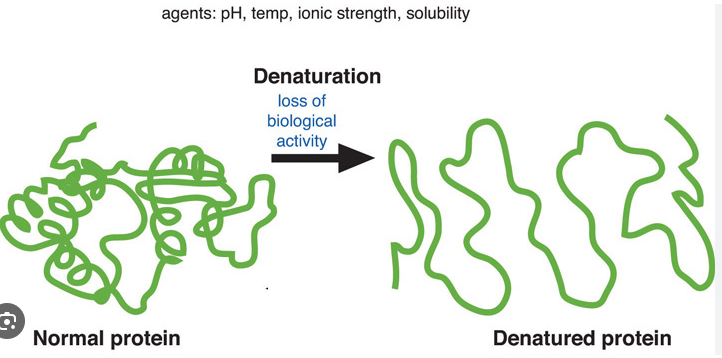
Protein Denaturation
Results from changes in the 3D shape caused by temperature, PH or ionic concentration changes
Protein unravels and looses conformation
If peptide bonds break the protein is destroyed
Enzymes function best within a narrow range of the above conditions
Nucleic Acids
Assembly instructions for all proteins in living organisms
Includes DNA and RNA
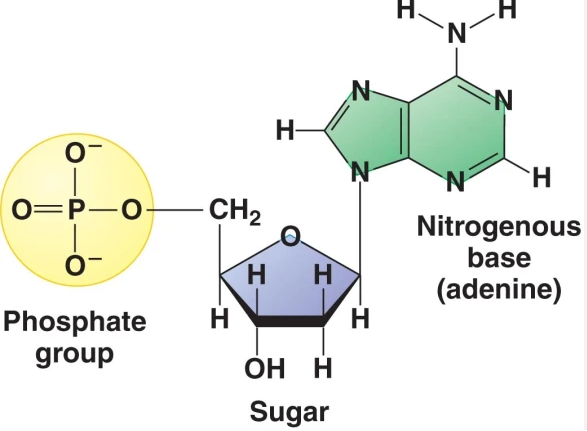
DNA and RNA are polymers that are made up of monomer units, these are called nucleotides
Nucleotides have three parts:
Nitrogenous base
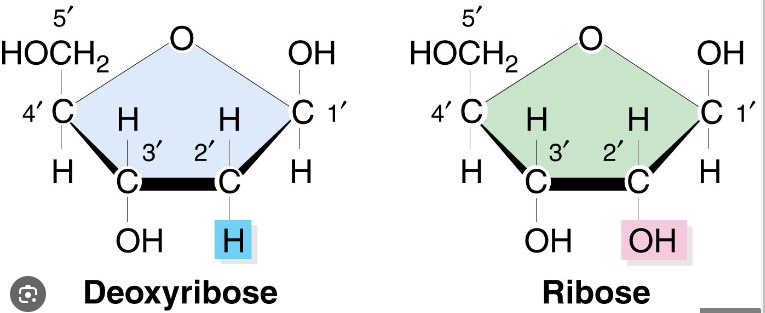
5-C sugar
Phosphate group (s)
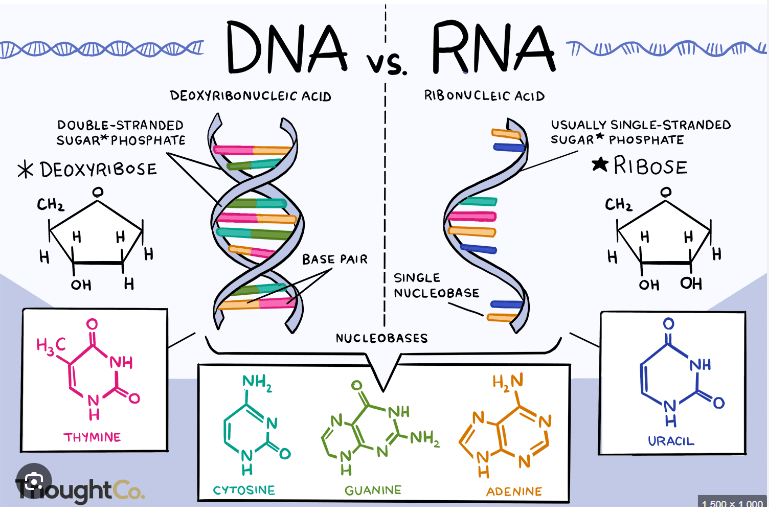
DNA
Source of genetic information for every living organism
Directs all cellular activities and is able to replicate itself so that new cells and organisms can be created
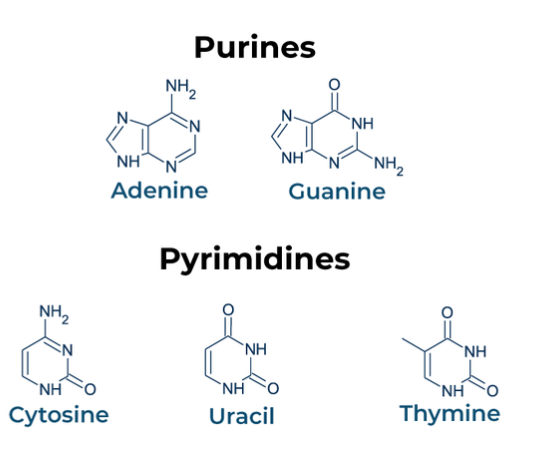
Nitrogenous Base
Divided into two groups based upon the number of rings in the structure
Purines: Two rings, adenine and guanine
Pyrimidines: One ring, thymine and cytosine
Linkage and Phosphodiester Bonds
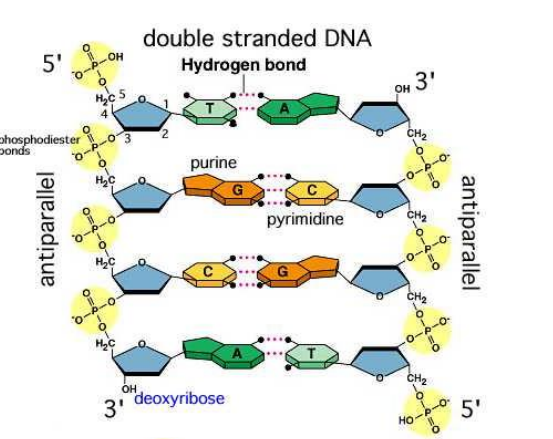
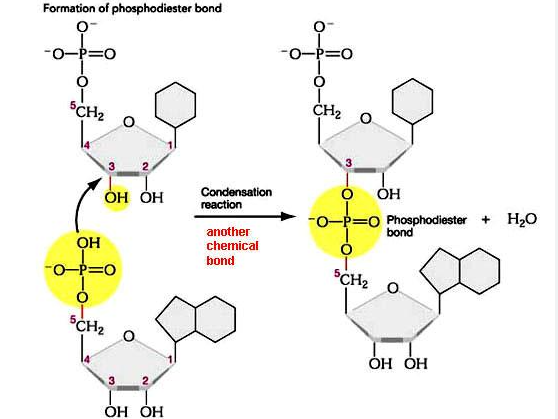
DNA nucleotides are joined together at the phosphate group through phosphodiester bonds between carbon 5 of one molecule to the hydroxyl group at carbon 3 from another molecule
Additional nucleotides are always added in the 3 end of the previous nucleotide

Hydrogen Bonds
DNA is a double stranded molecule where the 2 strands run anti-parallel to each other
Hydrogen bonds unite strands of DNA together
Adenine will always bind to thymine by 3 hydrogen bonds
Guanine and cytosine will always bond together by 3 hydrogen bonds
A purine will only pair up with its complementary pyrimidine
RNA
Single stranded polymer
Involved in protein synthesis
Composed of:
Ribose sugar
Phosphate group
4 nitrogen containing bases (C, G, A and U)
All of the bases are the same as those found in DNA except Uracil (uracil replaces thymine in RNA)
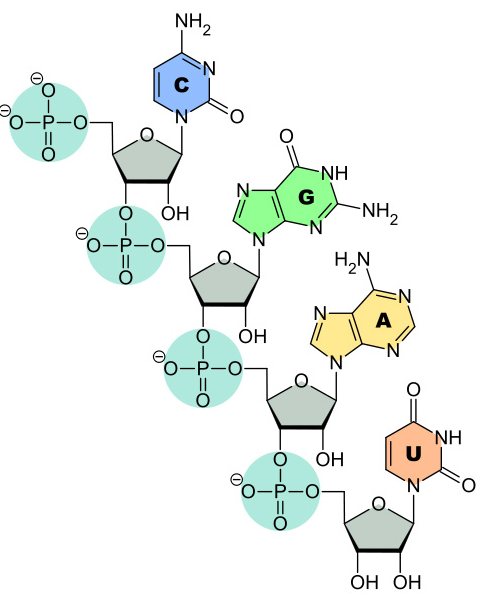
RNA Linkages: Phosphodiester Bonds
Also synthesized in the 5’ to 3’ direction in a condensation reaction
A phosphodiester bond forms between phosphate group from one nucleotide and the hydroxyl group from the second nucleotide
Deoxyribose vs. Ribose
DNA vs. RNA
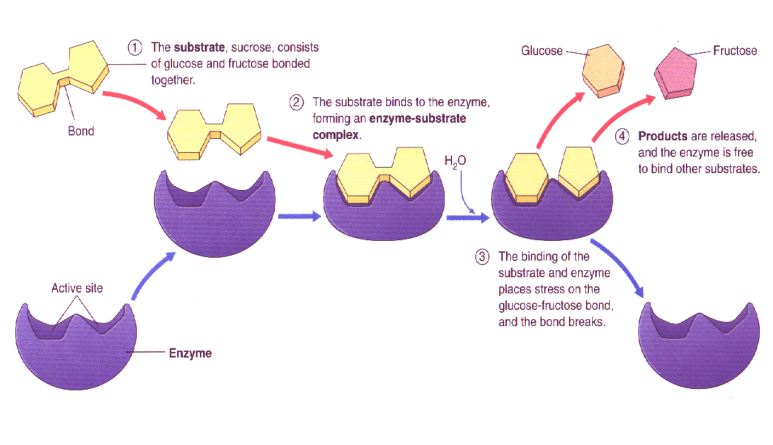
What is an Enzyme?
Biological catalysts that increase the speed of biochemical reactions within cells.
Enzymes are proteins that are NOT consumed during reactions
They can catalyze the same reaction repeatedly
Each enzyme has a unique shape, which determines which reactions it catalyzes
Induced Fit Hypothesis
Initially the active site is not a direct fit for the substrate
Just prior to substrate binding, the enzyme modifies its shape to better accommodate the substrate
The enzyme binds to the substrate
Creates an enzyme-substrate complex
Enzyme converts substrate into product(s)
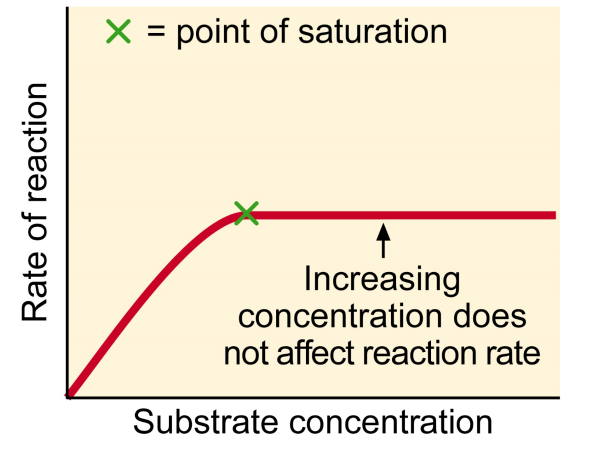
List the factors that affect Enzyme Activity
Enzyme and Substrate Concentration
Enzyme Inhibitors
pH and Temperature
Enzyme and Substrate Concentration
If excess substrate, rate of reaction becomes proportional to enzyme concentration
If enzyme at a constant concentration, increasing substrate concentration will only increase reaction rate to a point called saturation level
At this point, all enzyme molecules saturated with substrate
What are Enzyme Inhibitors? List the two types of inhibition
Molecules that bind to an enzyme and lowers the rate at which it catalyzes a reaction
Competitive and Non-Competitive
Competitive Inhibitors
Compete with the substrate for the enzyme’s active site
Shape/Mimics substrate
Enter the enzyme’s active site and prevent the normal substrate from binding
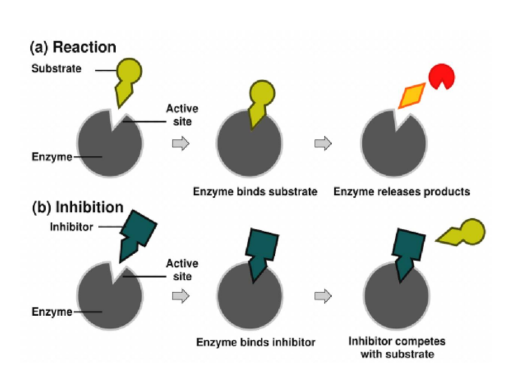
Non-Competitive Inhibitors
Does not compete for active site
Attaches to enzyme on a site other than the active site (allosteric site)
Causes the enzyme to change shape, so that active site looses affinity for its substrate
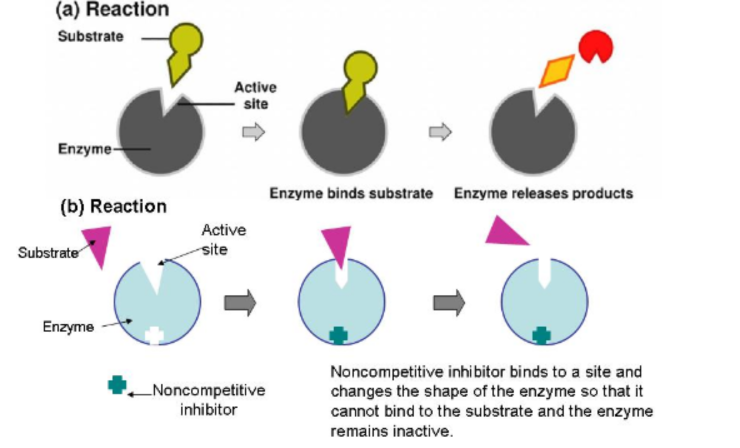
Allosteric Regulation
Allosteric regulation is a mechanism by which a molecule binds to an enzyme at a site other than its active site, called the allosteric site, altering the enzyme's activity.
This binding can either inhibit or activate the enzyme, depending on the nature of the regulator.
Regulatory molecules bind to a site located far from the active site called an allosteric site
Allosteric activator: keeps the active site of an enzyme available to its substrate
Allosteric inhibitor: Is a non-competitive inhibitor
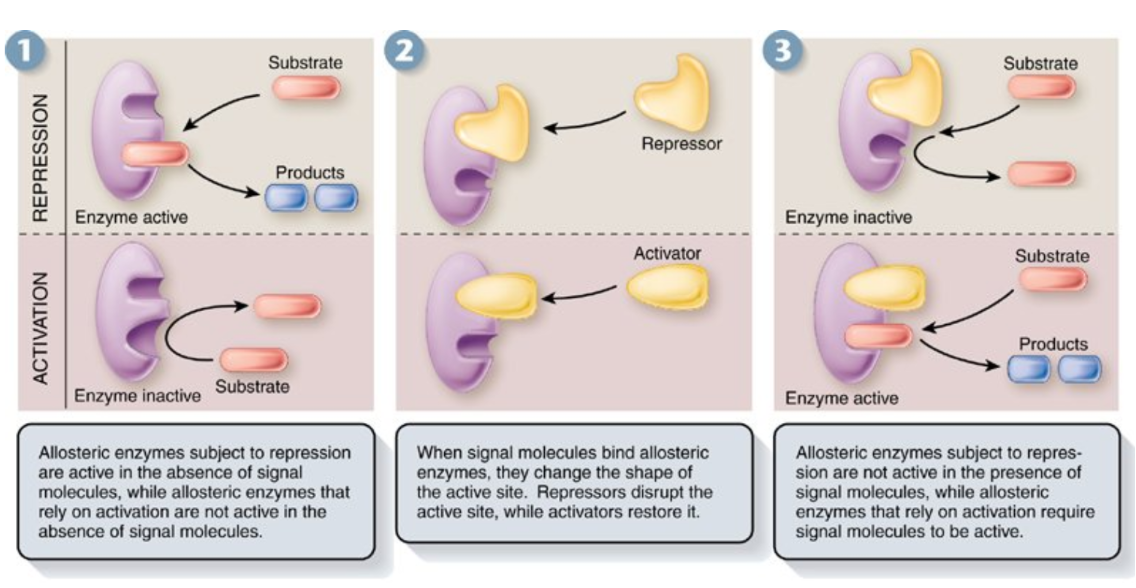
Feedback Inhibition
A product of a reaction acts as a regulator of the reaction
Used by cells to control metabolic pathways involving series of reactions
If the product accumulates in excess, its effect as an inhibitor automatically slows or stops the enzymatic reaction that produces it.
If the product is scarce, the inhibition is reduced, and the rate of the reaction increases.
Product formed later in sequence allosterically inhibits the enzyme catalyzing the first reaction of the pathway
Each reaction catalyzed by specific enzyme
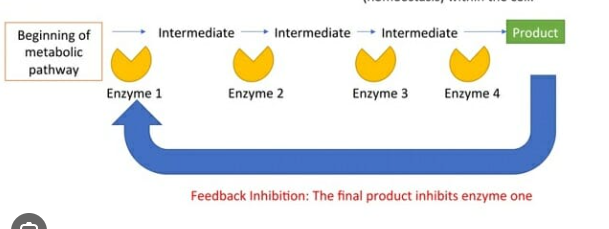
Temperature
As temperature increases beyond a critical point, enzymes denature
Every enzyme has an optimal temperature at which it works best (humans -37 C)
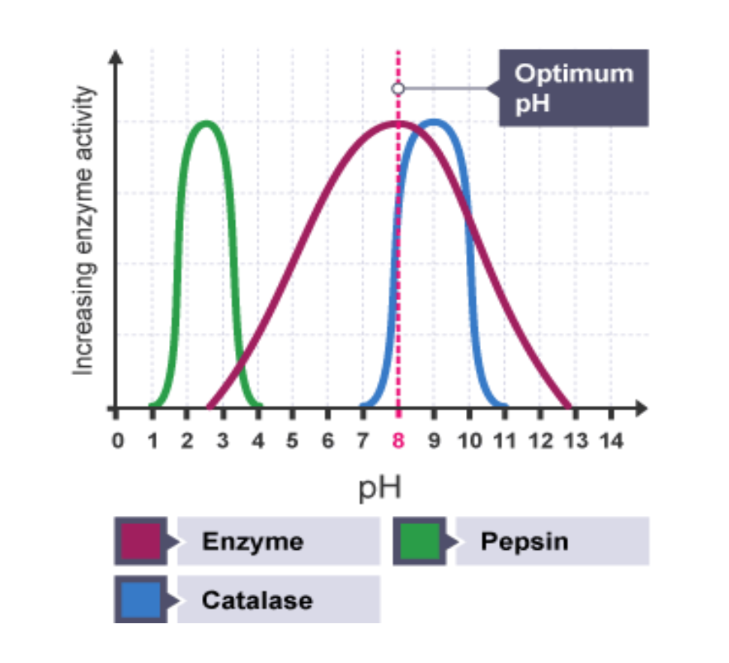
pH
Enzymes have optimal pH range
Eg. Pepsin works best at pH of 2 in stomach, inactive in small Intestine pH of 8
The Cell Membrane
Separates the living cell from nonliving surrounding
Selectively Permeable - Controls which substances can cross the membrane, allows some substances to cross more easily than others
Keep nutrients in and waste products out
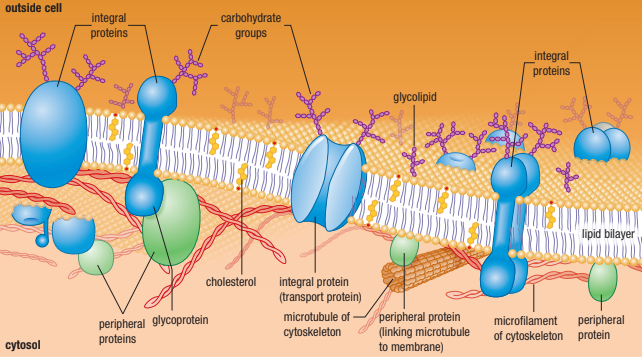
The Fluid Mosiac Model
Membranes are not rigid, with molecules locked in place
Instead molecules are in constant motion (Fluid Part)
Membrane consists of a fluid phospholipid bilayer - proteins embedded into it float freely
There are many types of proteins, lipids and carbohydrates embedded in the membrane (Mosiac Part)
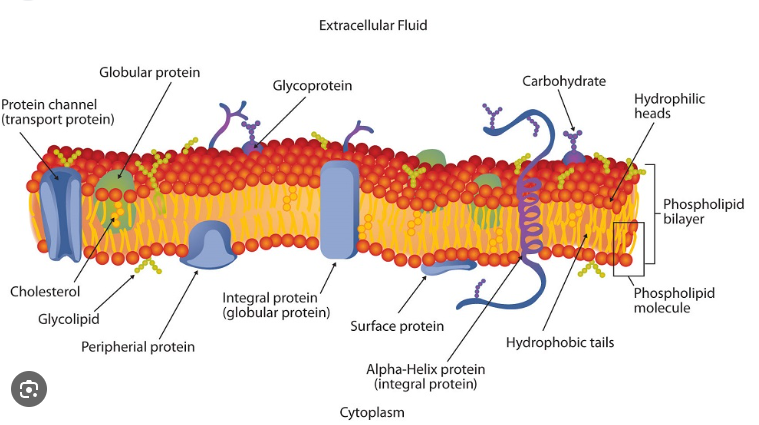
Phospholipids
Phosphate Group Head (Hydrophilic Polar Head)
2 Fatty Acid Tails (Hydrophobic Non-Polar Tails)
Forms a lipid bilayer in aqueous (watery environments) that is two lipid molecules thick
No water inside the lipid bilayer itself - water is present outside and inside the cell
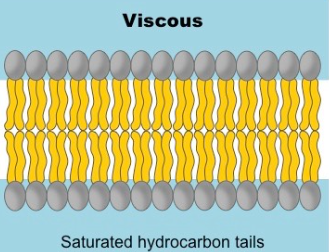
Fluidity - Saturated Fatty Acids
Saturated hydrocarbons - each carbon is bound to the maximum number of hydrogen atoms
Single bounds cause the membrane to form a semi solid gel due to linear arrangement
Have a straight shape - lipids are able to pack together more tightly
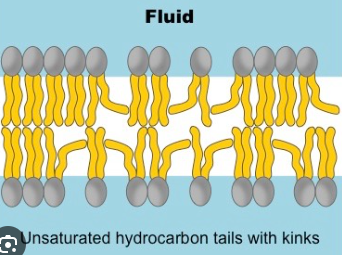
Fluidity - Unsaturated Fatty Acids
Double bonds in an unsaturated fatty acid bend its structure - lipid molecules are less straight and more loosely packed
Double bonds keep membrane fluid (less viscous)
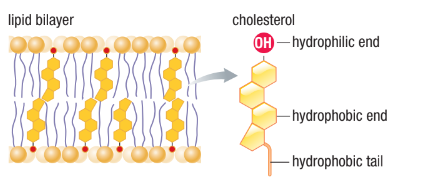
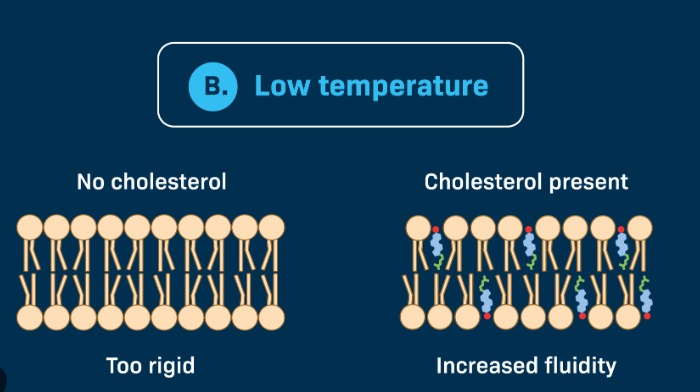
Cholesterol
A type of sterol (steroid with OH group at one end and hydrocarbon chain at the other)
Plays a role in membrane fluidity
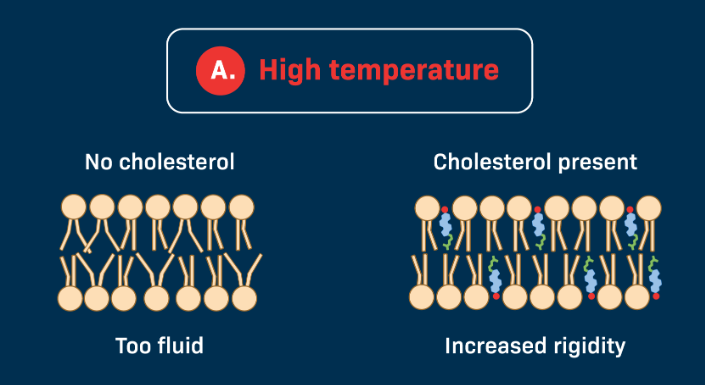
If temperature is High
Phospholipids move quickly and membrane may become too fluid
Cholesterol helps to restrain the movement of the lipid molecules in a membrane thus reducing the fluidity of the molecules
If Temperature is Low
Phospholipids become tightly packed and membrane may become a highly viscous semi-solid gel
Cholesterol occupies space between phospholipids
Keeps the oil bilayer flexible in cold temps - prevents fatty acids from associating and forming a non-fluid gel
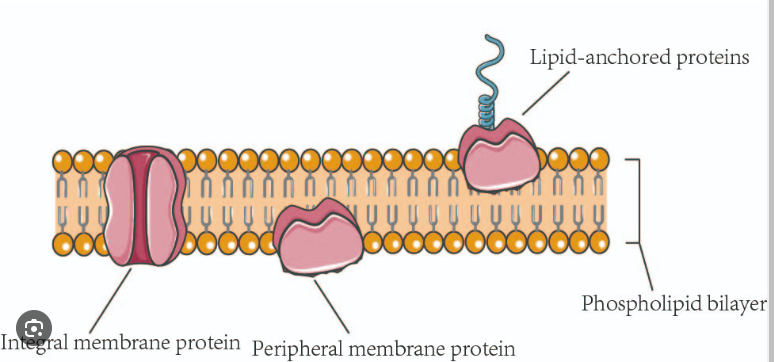
Integral vs Peripheral Membrane Proteins
All membrane proteins can be separated into these two additional categories
Integral Membrane Protein → Embedded in the lipid bilayer
Peripheral Membrane Protein → On the surface of the membrane
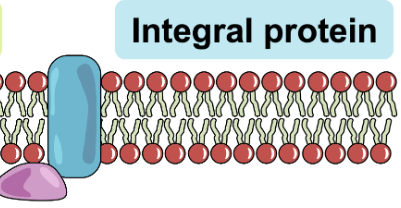
Integral/Transmembrane Proteins
Span the entire bilayer and are exposed to the aqueous environment on both sides of the membrane
Within Membrane - Non polar amino acids are hydrophobic which matches the hydrophobic region of the phospholipid tails
Outer Membrane - Polar amino acids are hydrophilic and extend into the extracellular fluid on the outside and into the cytoplasm on the inside
Transport Proteins → Acts as channels or pumps
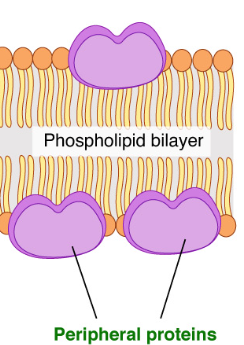
Peripheral Proteins
Loosely bound to the surface of the cell membrane
Do not interact with the hydrophobic core of the membrane
Outer Surface - They hold onto the surface of the membrane with ionic and H-bonds. Most are on the extracellular side of the membrane, but some are on the cytoplasm side as well.
Act as cell identity markers (antigens) or receptors
Inner Surface - Anchor points for microtubules or microfilaments
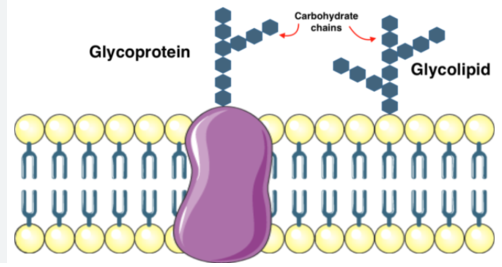
Membrane Carbohydrates
Some of the membrane lipids and proteins have carbohydrates linked to them.
Called glycolipids (any membrane lipid + carbohydrate) or glycoproteins (membrane component with sugar or carb + aa)
Crucial for cell-cell recognition and signaling
Allows the cell to distinguish one cell from another and to identify foreign cells or particles (bacterial or viral infections)
Recognize and bind to carbohydrate receptors on adjacent cells and leads to attachment between cells
Passive Transport
The movement of a substance across a membrane without the need to expend chemical energy (ATP)
Universe tends towards disorder (entropy)
If molecules are more concentrated on one side of a membrane, they will become equally distributed on both sides until equilibrium is reached
What are the three types of passive transport?
Simple Diffusion
Facilitated Diffusion
Osmosis
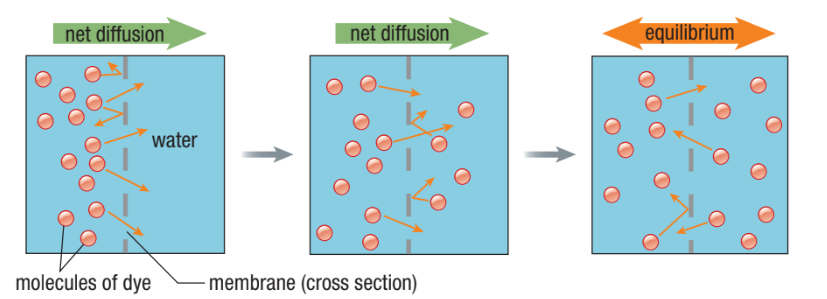
Simple Diffusion
The ability of a substance to move across a membrane unassisted
Movement of a substance from high to low concentration (no energy needed)
Rate of diffusion depends on the concentration gradient between two sides of a membrane
Dynamic equilibrium - even after the concentration of molecules is the same on both sides, they continue to move from one side to the other
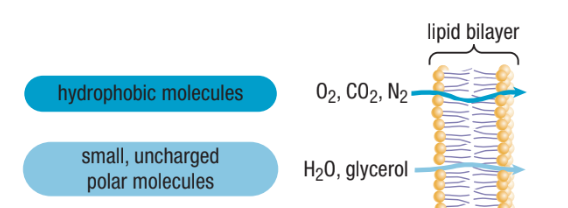
What can Diffuse Through The Phospholipid Bilayer?
Very small non-polar molecules can get through directly (eg. oxygen gas and carbon dioxide)
Small, uncharged polar molecules (eg. water and glycerol can also cross easily)
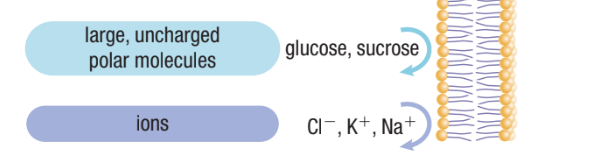
What Molecules Cannot Can’t Through Directly?
Ions (positively charged Cl, negatively charged K and positively charged Na)
Large uncharged polar molecules (polysaccharides and proteins)
Facilitated Diffusion
Diffusion through transport protein channels
Channels help move specific molecules across cell membrane
Still driven by concentration gradient (high to low concentration)
In what two ways does the membrane become semi-permeable?
Channel Proteins
Carrier Proteins
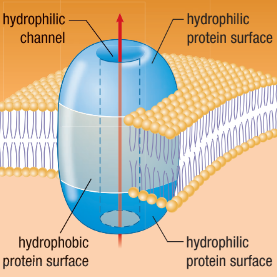
Channel Proteins
A hydrophilic pathway in a membrane that enables water and ions to pass through
Open tunnel
Specific channels allow specific material across cell membrane
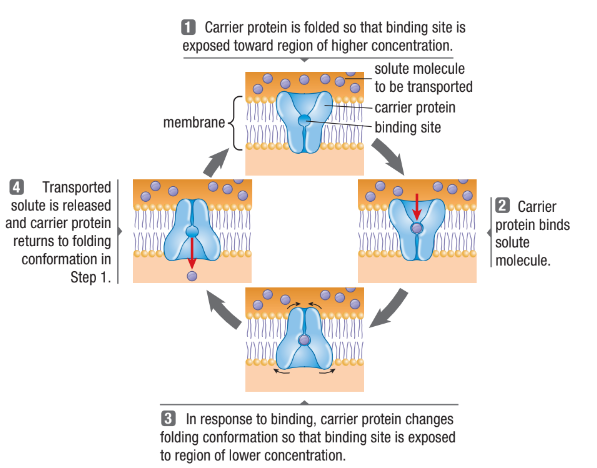
Carrier Proteins
Protein changes shape and allows the solute to enter/exit the cell
Form passageways through the lipid bilayer
Each binds to a specific solute and transports it across the bilayer
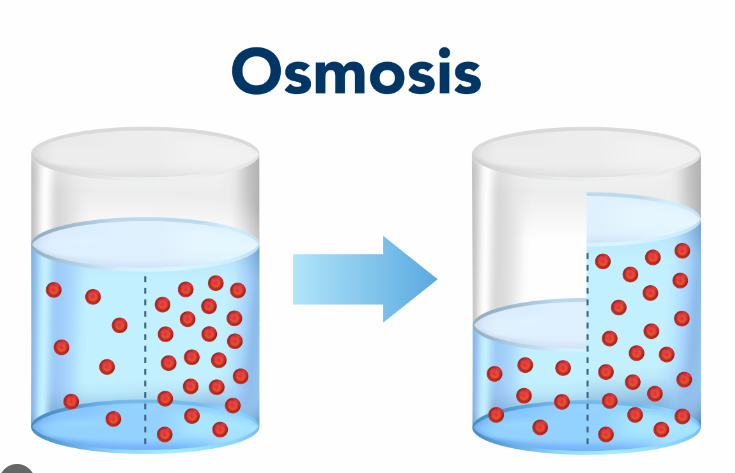
Osmosis
Diffusion of water from high concentration of water (low amount of solute) to low concentration of water (high amount of solute).
Water will move to the more concentrated side (more solute) to balance it out.
Across a semipermeable membrane
Water will always chase the hypertonic side (high amount of solute)
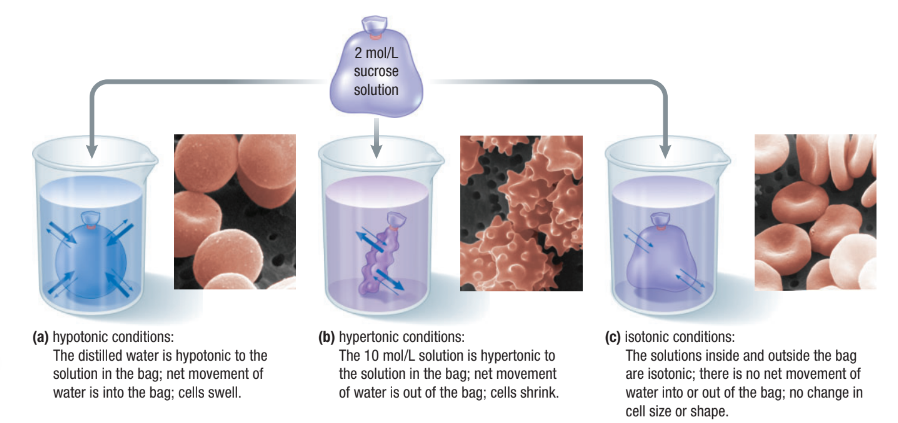
Concentration of Water
Direction of osmosis is determined by comparing total solute concentrations
Hypertonic: More solute, less water
Hypotonic: Less solute, more water
Isotonic: Equal solute, equal water
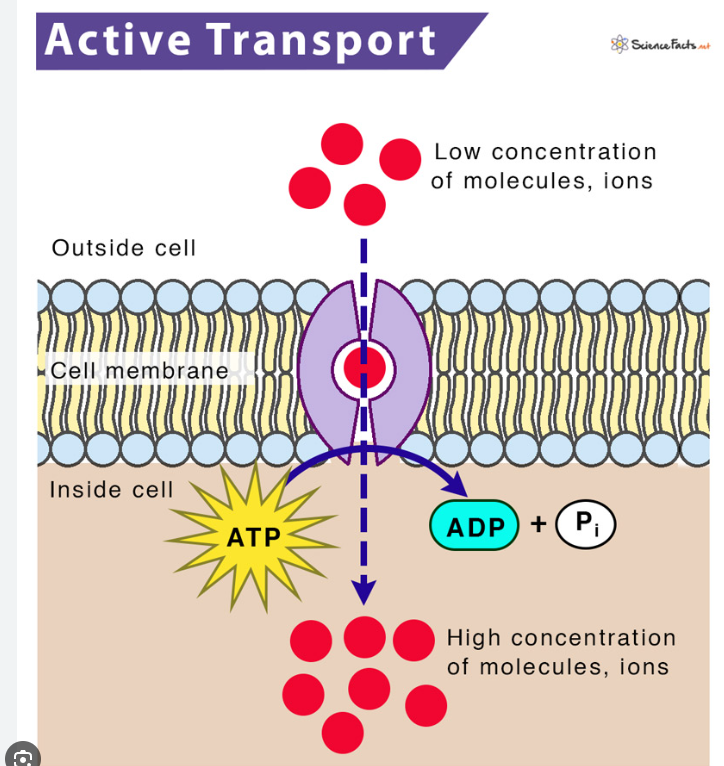
Active Transport
Cells may need to move molecules against concentration gradient
From low concentration to high concentration
Use of protein “pumps”
The term “active” refers to the fact that the cell has to expend energy = ATP
What are the two types of active transport?
Primary Active Transport
Secondary Active Transport Pumps
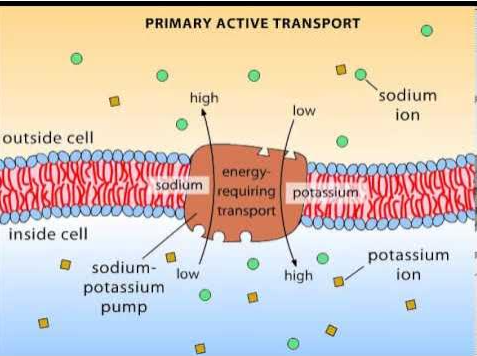
Primary Active Transport
Pumps (carrier proteins) that move positively charged ions across the membrane
Electrochemical Gradient:
Voltage across a membrane is a difference in electrical charge on either side of a membrane
Forms as a result of many positive cations on one side of a membrane compared to the other
Both the voltage difference and difference in ion concentration creates an electrochemical gradient
It is a form of stored potential energy which is used in nerve impulse transmission or to make ATP
Example of Primary Active Transport
Sodium-potassium pump (Na+/K+ pump)
Transports sodium ions (Na+) out of the cell and potassium ions (K+) into the cell, both against their concentration gradients.
This process is essential for maintaining cell membrane potential and regulating cell volume and ion balance.
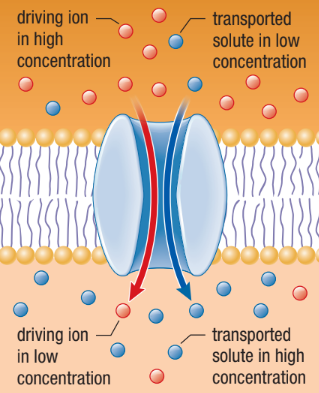
Secondary Active Transport Pumps
Uses the concentration gradient of an ion set up by a primary active transport pump as its energy source
As the ion flows back along its concentration gradient, it brings a second molecule/ion along with it
Symport
A solute that moves through the membrane channel in the same direction as the driving ion
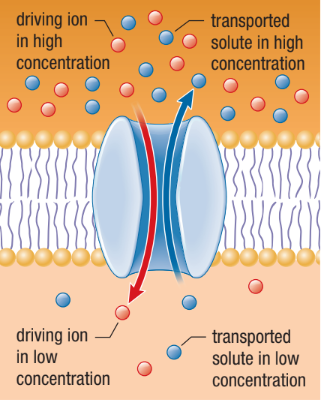
Antiport
The driving ion moves through the membrane in one direction providing energy for the transport of another molecule in the opposite direction
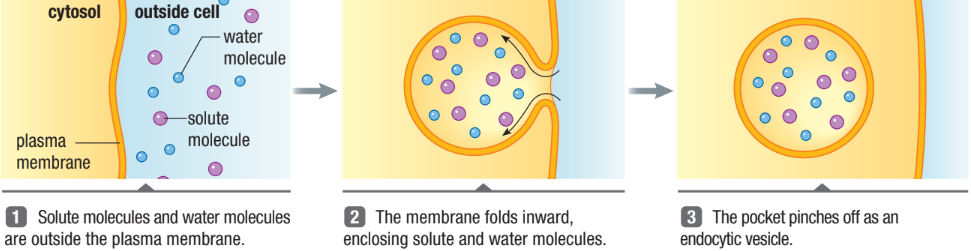
How are large molecules moved in and out of the cell?
Through vesicles and vacuoles
Endocytosis
Phagocytosis: “cellular eating”
Movement of large molecules or whole cells engulfed a cell
Pinocytosis: “cellular drinking”
Transports liquids into cell within vesicles
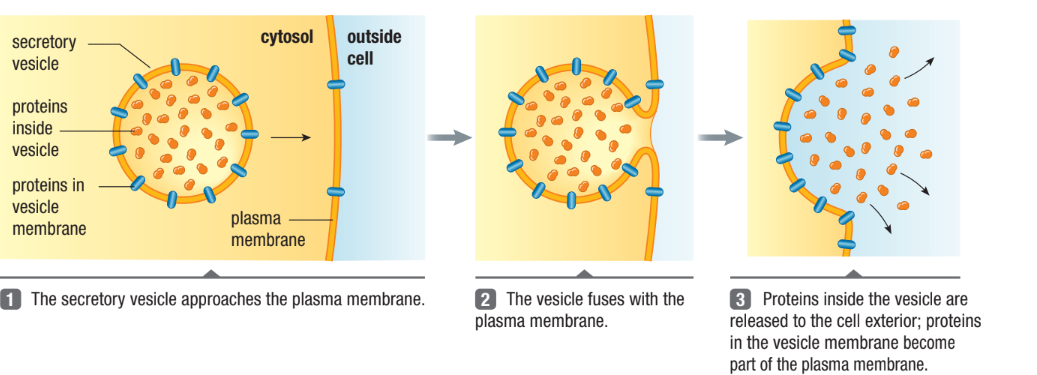
Exocytosis
“Exit”
Exports large molecules out of the cell