Zeff and Periodic Trends (Ch. 6)
0.0(0)
Card Sorting
1/10
Last updated 7:13 PM on 9/20/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
1
New cards
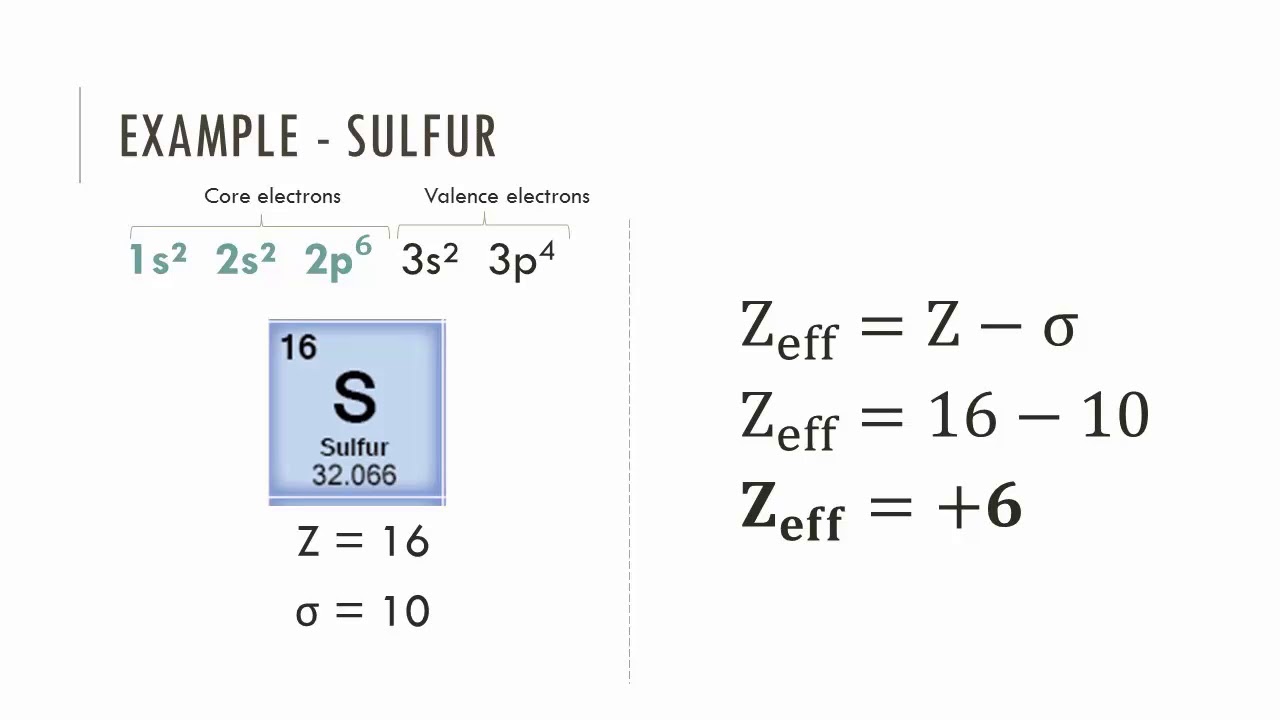
What does Zeff mean?
Effective nuclear charge – the measure of the pull felt by valence electrons
2
New cards
How do you calculate Zeff?
Zeff = atomic number - number of shielding electrons
3
New cards
When talking about noble gases and periodic trends, which trends do noble gases get excluded from?
Reactivity (because noble gases are nonreactive) and electronegativity (because they have a full valence shell and don't tend to share electrons with other atoms)
4
New cards
What is atomic radius?
The atomic radius is the distance from the nucleus to the valence electrons
5
New cards
What is ionization energy?
The amount of energy needed to remove a valence electron from an atom. This is a measure of how strongly the nucleus holds on to the outer electrons.
6
New cards
What is electronegativity?
A measure of how much an atom “hogs” electrons when it is bound to other atoms with a chemical bond. The more electronegative the atom, the more it will hog the electrons.
7
New cards
Reactivity trends for metals and nonmetals
For metals: down and left increases (because for metals the less well they hold on to electrons = more reactive)
For nonmetals: up and right increases (because for nonmetals the better and an element holds on to electrons = more reactive)
For nonmetals: up and right increases (because for nonmetals the better and an element holds on to electrons = more reactive)
8
New cards
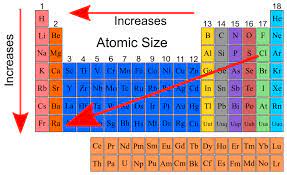
Trend arrow for atomic radius and why
Starts in top right and points to bottom left because as you go down in a group there are more shells so the distance is greater, and as you move across in a period the hold is stronger on the valence electrons which pulls them closer to the nucleus. The biggest radius would be the most shells with the weakest pull on the electrons.
9
New cards
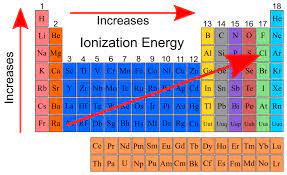
Trend arrow for ionization energy and why
Arrow starts in bottom left and points to the upper right because the smaller the atomic radius and the stronger the hold on the valence electrons, the harder it will be to remove valence electrons.
10
New cards
Trend arrow for electronegativity and why
Arrow goes from bottom left to upper right because as you move across a period the Zeff increases which is the hold on the valence electrons, and that means that if the atom got more electrons in a chemical bond it would be very grabby. It decreases as you go down a group because the distance between the electrons and the nucleus is greater, which makes it harder for the atom to be grabby on electrons.
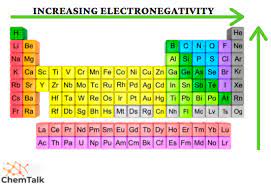
11
New cards
Most reactive nonmetal
FLUORINE! Be careful to ignore the noble gas row :)