equations
0.0(0)
Card Sorting
1/16
Earn XP
Description and Tags
Last updated 10:34 AM on 5/21/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
1
New cards
**Moles, mass and formula mass**
moles = mass/mr
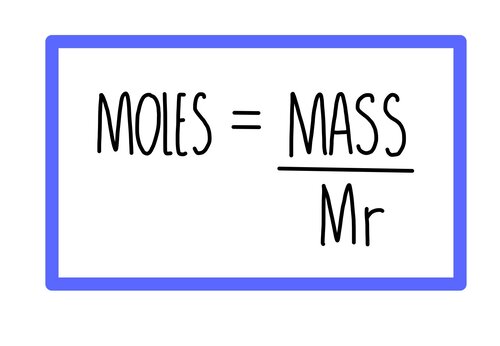
2
New cards
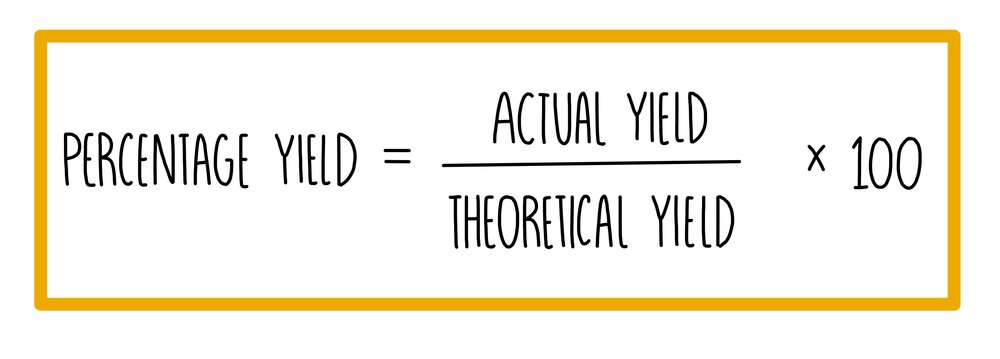
**Percentage yield**
* Percentage yield = (actual yield / theoretical yield) x 100
3
New cards
**calculating empirical formula from masses**
**Step 1**: Find the **moles** of each element using the equation **moles = mass / Mr.**
**Step 2**: **divide** each of the moles by the **smallest number (of moles)** calculated.
**Step 2**: **divide** each of the moles by the **smallest number** calculated.
**Step 2**: **divide** each of the moles by the **smallest number (of moles)** calculated.
**Step 2**: **divide** each of the moles by the **smallest number** calculated.
4
New cards
what is the empirical formula
The **empirical formula** is the **simplest whole number ratio** of atoms of each element in a compound.
5
New cards
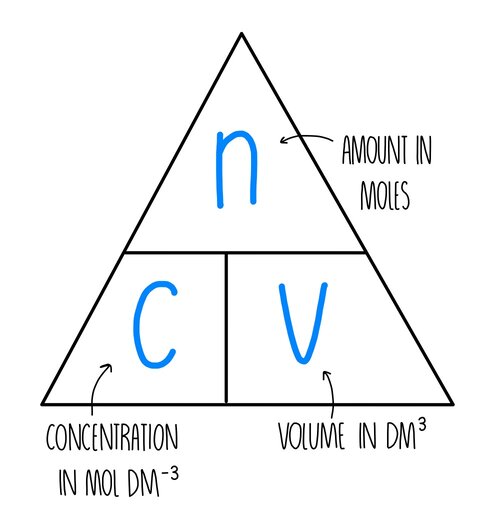
**Moles, concentration and volume**
moles = conc x volume
6
New cards
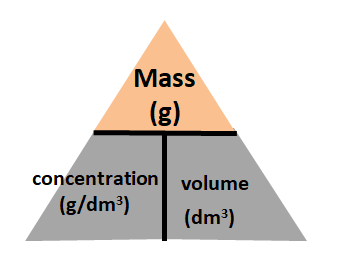
mass, concentration and volume
mass= conc x volume
7
New cards
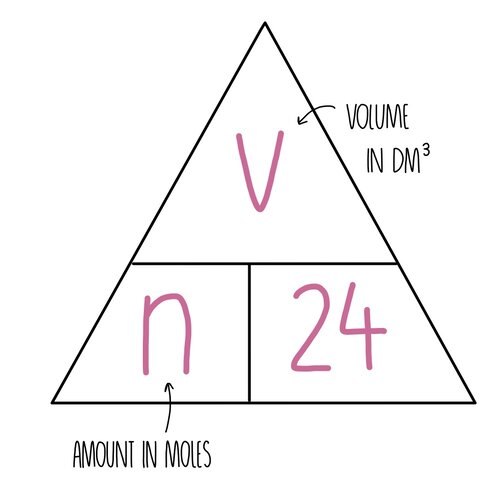
**Molar volume of a gas**
volume of a gas (in dm^3) = moles x 24
8
New cards
1dm^3 in cm^3
1000
9
New cards
multiple for nano (n)
x10^-9
10
New cards
multiple for a micro(μ)
10^-6
11
New cards
multiple for a milli (m)
10^-3
12
New cards
multiple for a centi (c)
10^-2
13
New cards
multiple for a kilo (k)
10^3
14
New cards
multiple for a mega (M)
10^6
15
New cards
multiple for a Giga (G)
10^9
16
New cards
multiple for a tera (T)
10^12
17
New cards
how to find limiting reactant
1. find the moles of each reactant
2. find the molar ratio to a prodcut in the equation ( use the big numbers
3. find out how many moles each reactant will make of the product
4. therefore ------ is the limiting factor as it produces --- moles less than -----