PKA Values
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
30 Terms
Hydroiodic Acid
-10
Hydrobromic Acid
-9
hydrochloric
-6
Sulfuric Acid
-3
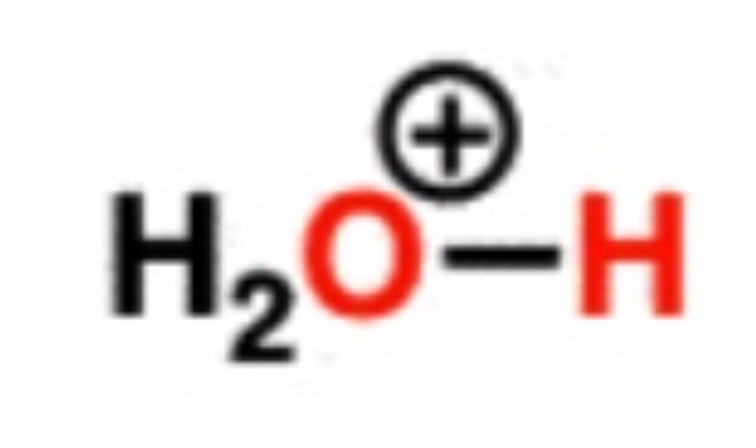
Hydronium ion
-7
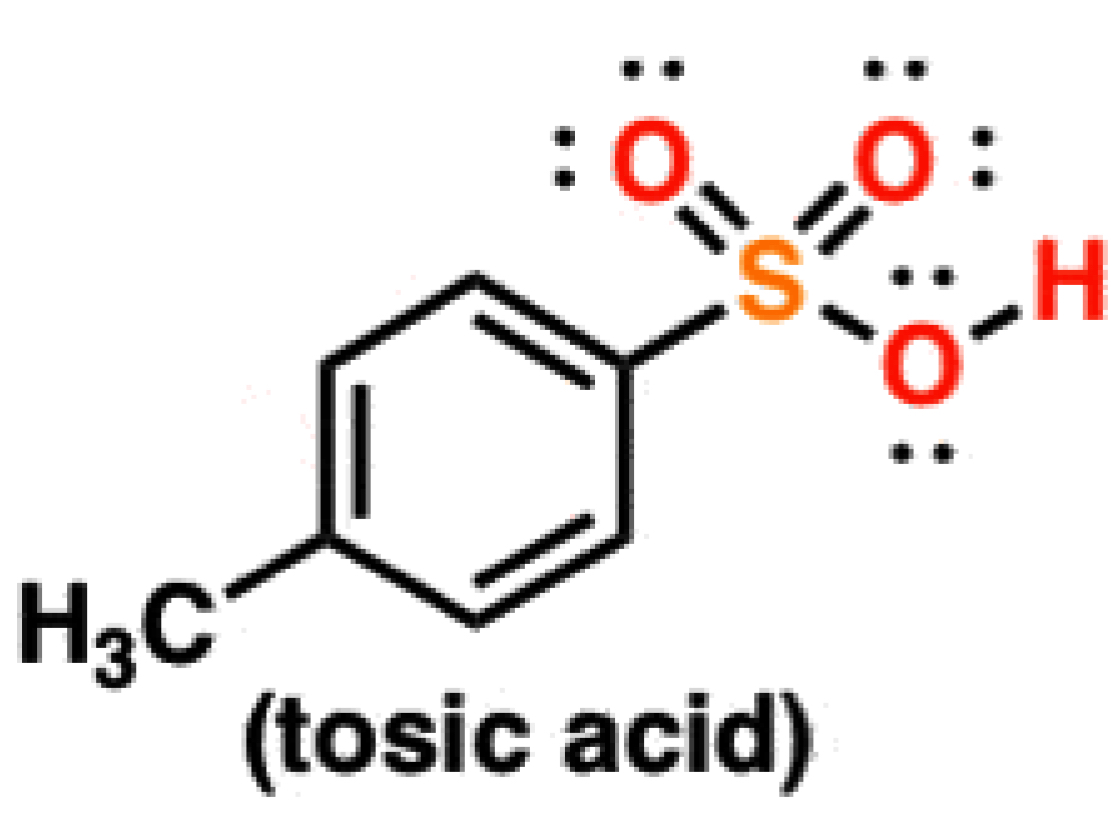
Sulfonic Acids
-1
Hydrofluoric Acid
3.2
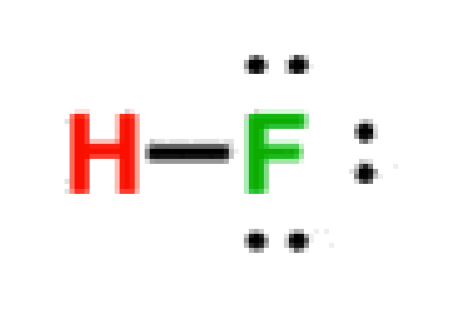
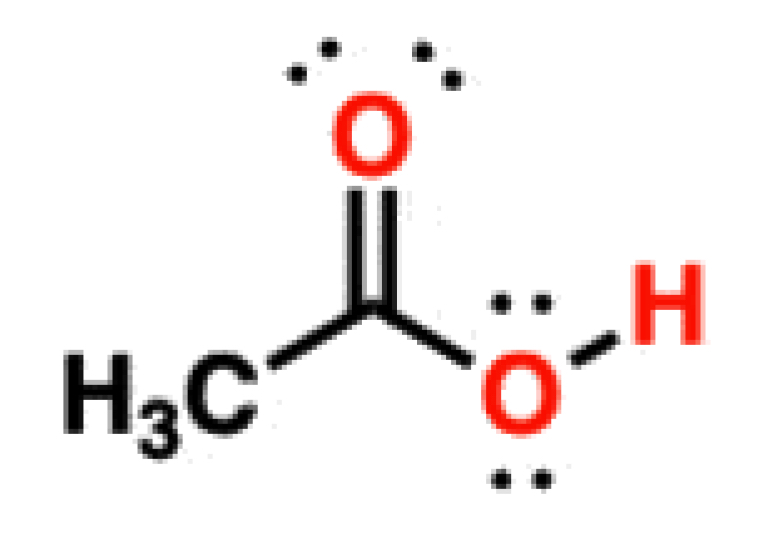
Carboxylic Acids
4
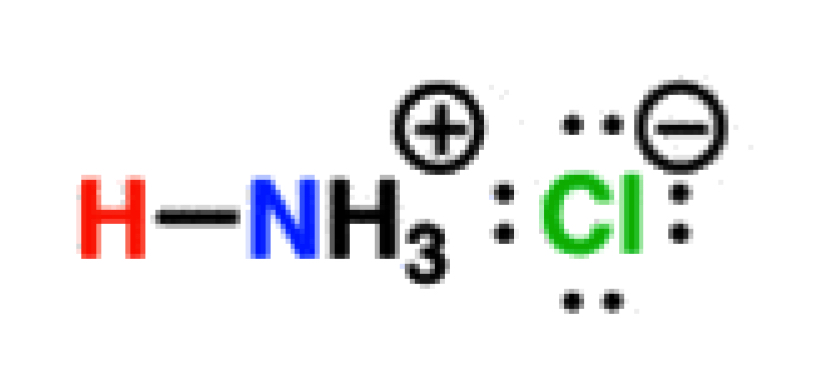
Protonated Amines
9-11
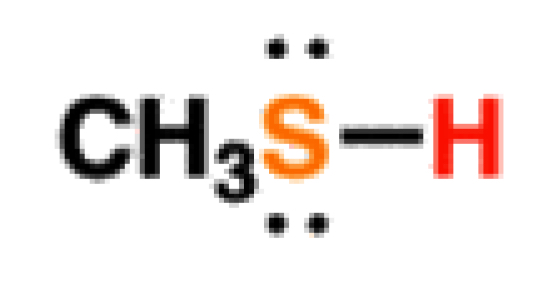
Thiols
13
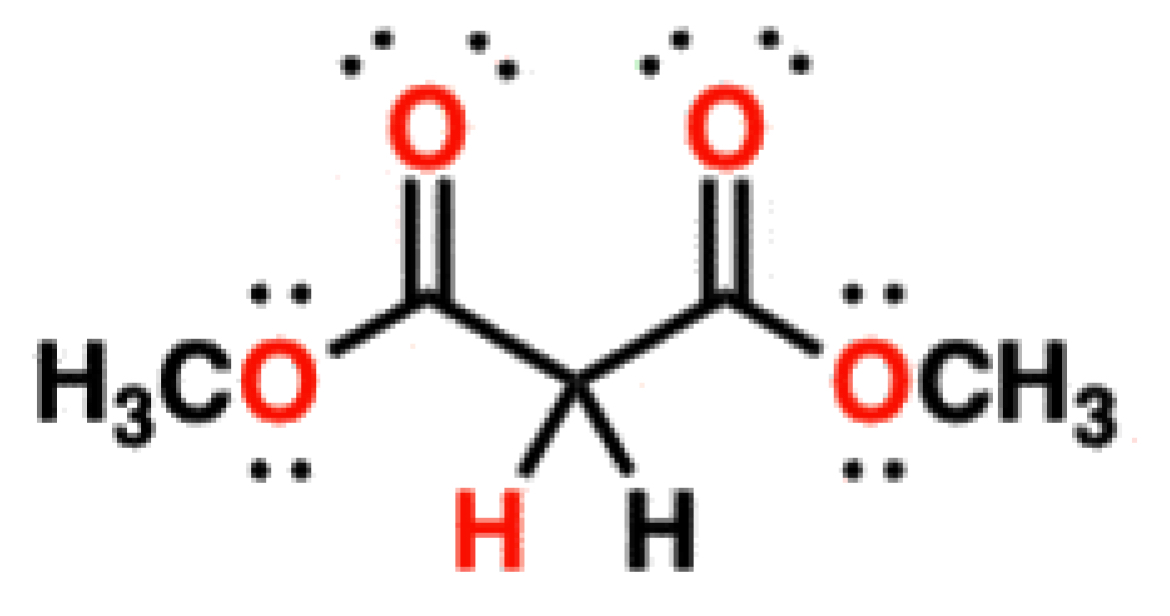
Malonates
13
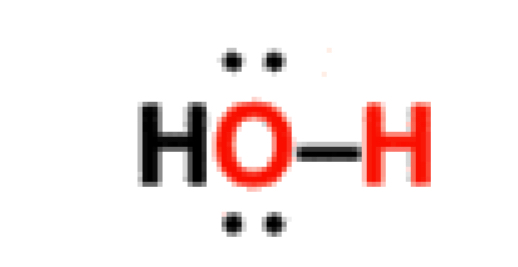
Water
14
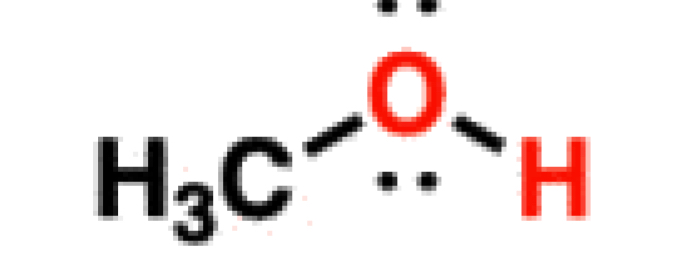
Alcohol
17
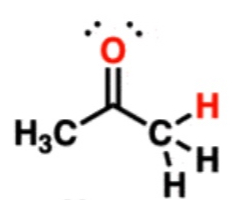
Ketone/Aldehyde
20-24
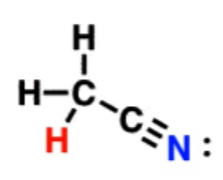
Nitrile
25
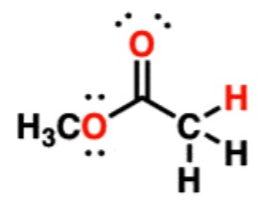
Ester
25
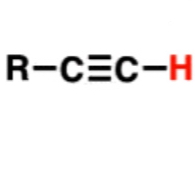
Alkyne
25
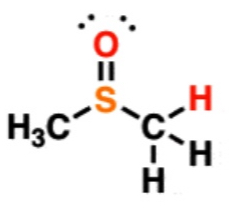
Sulfoxide
31
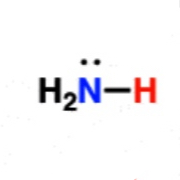
Amine
~35
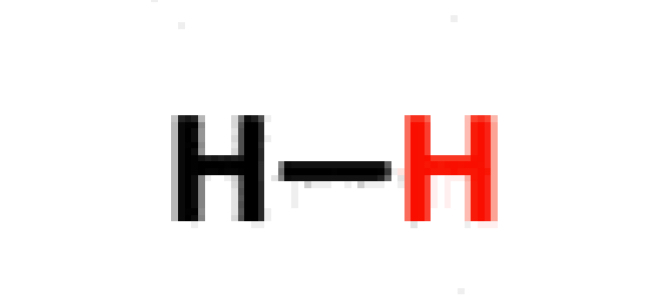
Hydrogen
36
Alkene
~44
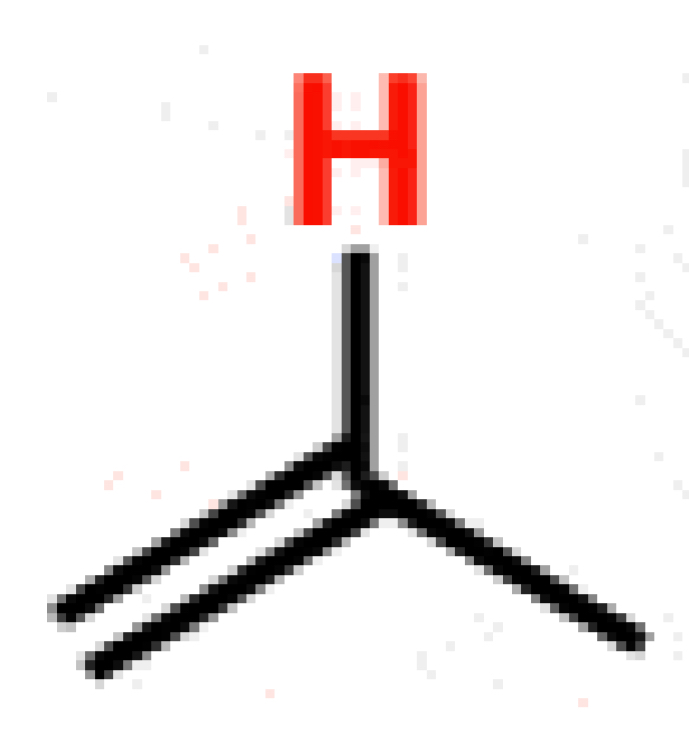
Alkane
~50 (>60)
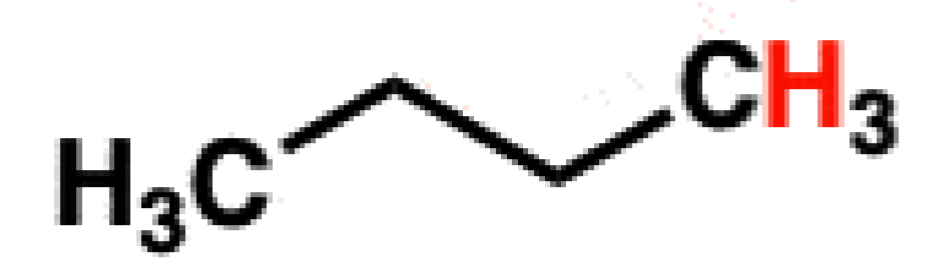
If there is a Higher PKA, what will the strength of the acid be?
Weak Acid
If there is a lower PKA, what will the strength of the acid be?
Stronger Acid
If there is a Strong Conjugate Base, What is it’s PKA?
What does PKA measure?
how tightly the proton is held by the bronsted acid. Higher PKA=proton held tighter
Bronsted Lowery Acid
Proton donor (gives off H+)
Bronsted Lowery Base
proton acceptor (accepts H+)
Lewis Acid
Electron pair acceptor (ex. what accepts the e- pair in arrow pushing)
Lewis Base
electron pair donor (ex. what donates the e- pair in arrow pushing)