Molecular Geometry
1/12
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
Linear
2 Bonds, 0 Lone Pairs, 180 degree bond angle, AB2 Type
Ex. BeCl2

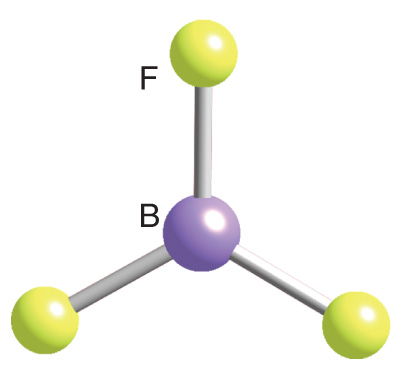
Trigonal Planar
3 bonds, 0 lone pairs, 120 bond angle, AB3 type
Ex. BF3
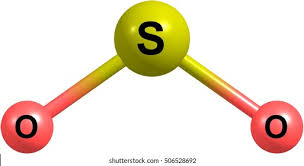
Bent/V Shaped
2 bonds, 1 lone pair, slightly less than 120 bond angle, AB2E type
Ex. SO2
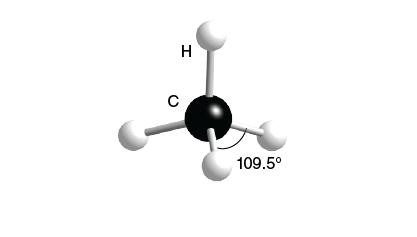
Tetrahedral
4 bonds, 0 lone pairs, 109.5 bond angle, AB4 type
Ex. CH4
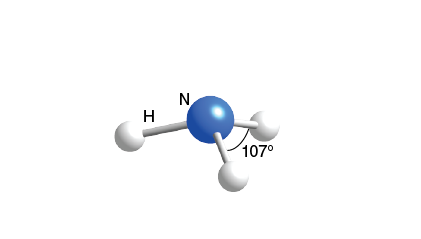
Trigonal Pyramidal
3 bonds, 1 lone pair, 107 bond angle, AB3E type
Ex. NH3
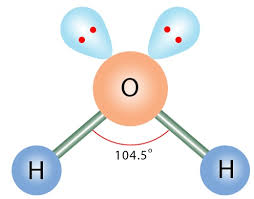
Bent/V-Shaped
2 bonds, 2 lone pairs, 104.5 bond angle, AB2E2 type
Ex. H2O
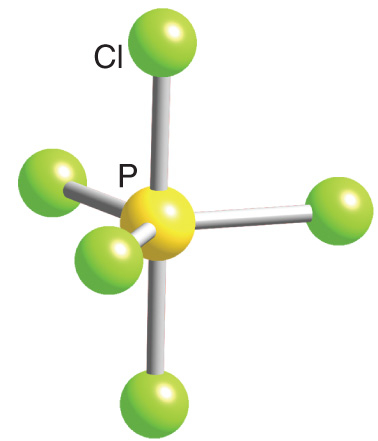
Trigonal Bipyramidal
5 bonds, 0 lones pairs, 120 in plane; 90 perpendicular to plane bond angle, AB5
Ex. PCl5
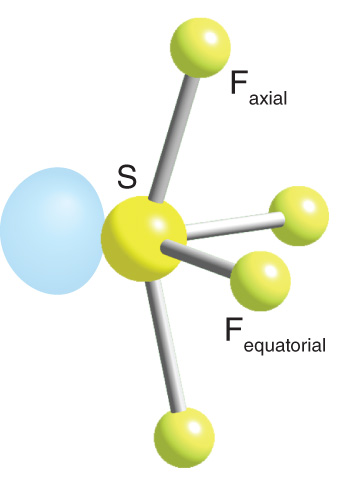
Seesaw
4 bonds, 1 lone pair, complex bond angle, AB4E type
Ex. SF4
T-shaped
3 bonds, 2 lone pairs, about 90 bond angle, AB3E2 type
Ex. ClF3
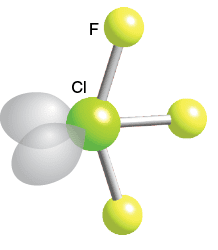
Linear
2 bonds, 3 lone paires, 180 bond angle, AB2E3 type
Ex. XeF2
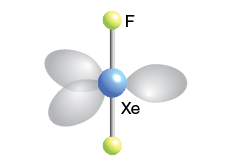
Octahedral
6 bonds, 0 lone pairs, 90 bond angle, AB6 type
Ex. SF6
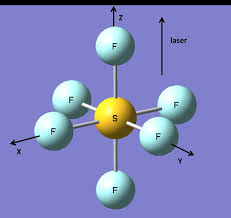
Square Pyramidal
5 bonds, 1 lone pair, about 90 bond angle, AB5E type
Ex. BrF5
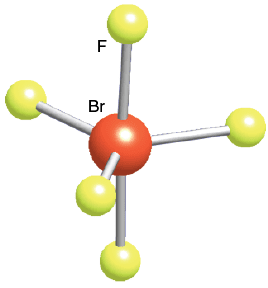
Square Planar
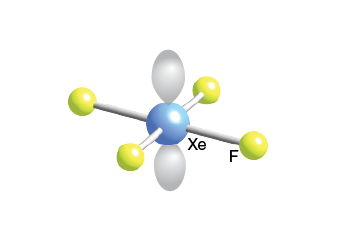
4 bonds, 2 lone pairs, 90 bond angle, AB4E2 type
XeF4