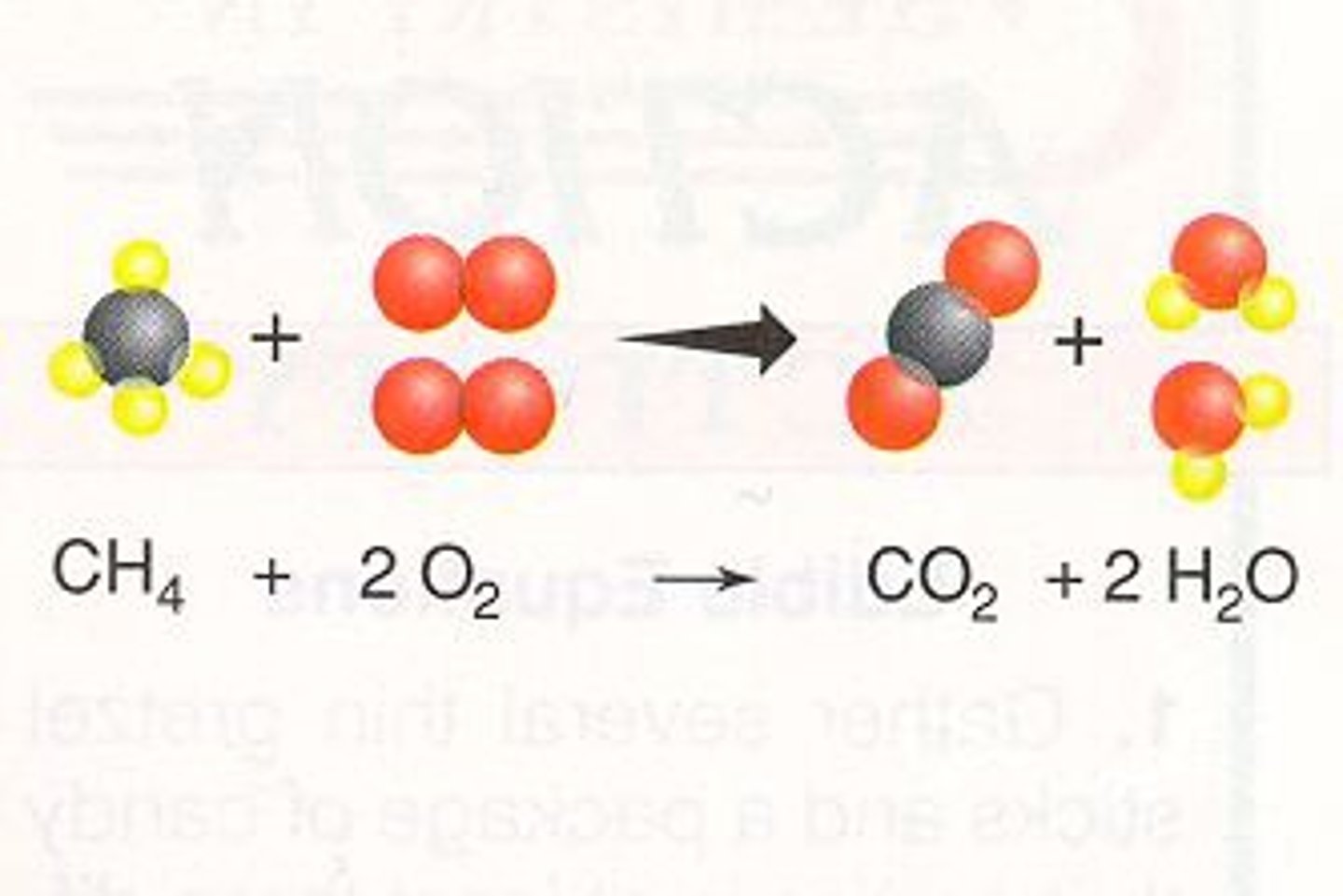Chemical Reactions and Equations
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
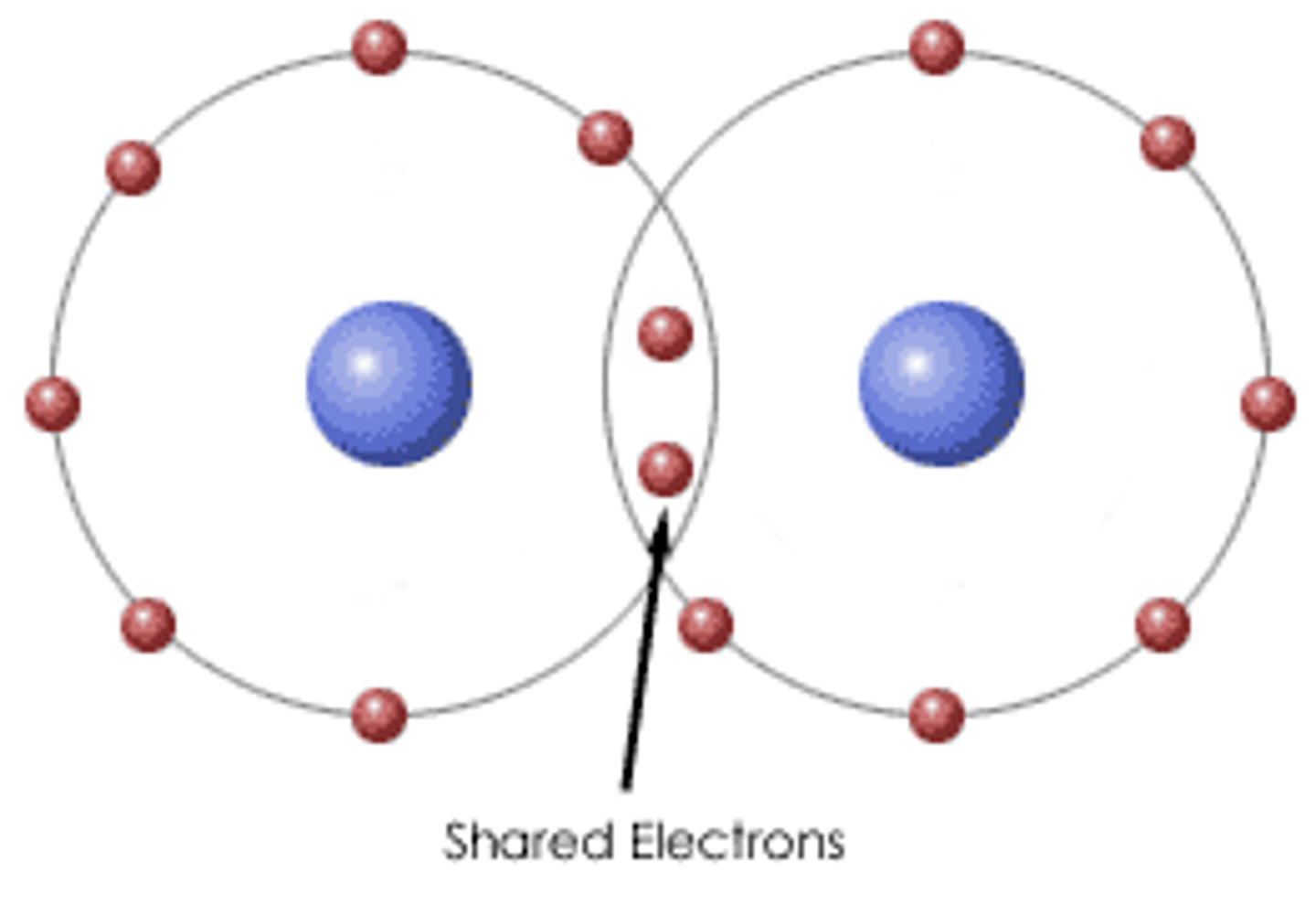
Chemical Bond
An interaction that holds two atoms together
Chemical Reaction
A process where one or more substances change to make one or more new substances

Precipitate
Solid substance formed in a solution

Chemical Properties
Characteristic properties that can be used to identify a pure substance; substance is changed during observation. Example: reactivity

Physical Properties
Characteristic property that can be used to identify a pure substance; substance is NOT changed chemically during observation. Example: state of matter

Chemical Formula
Shorthand way to write a compound's name using chemical symbols and subscripts; shows how many and what kind of elements
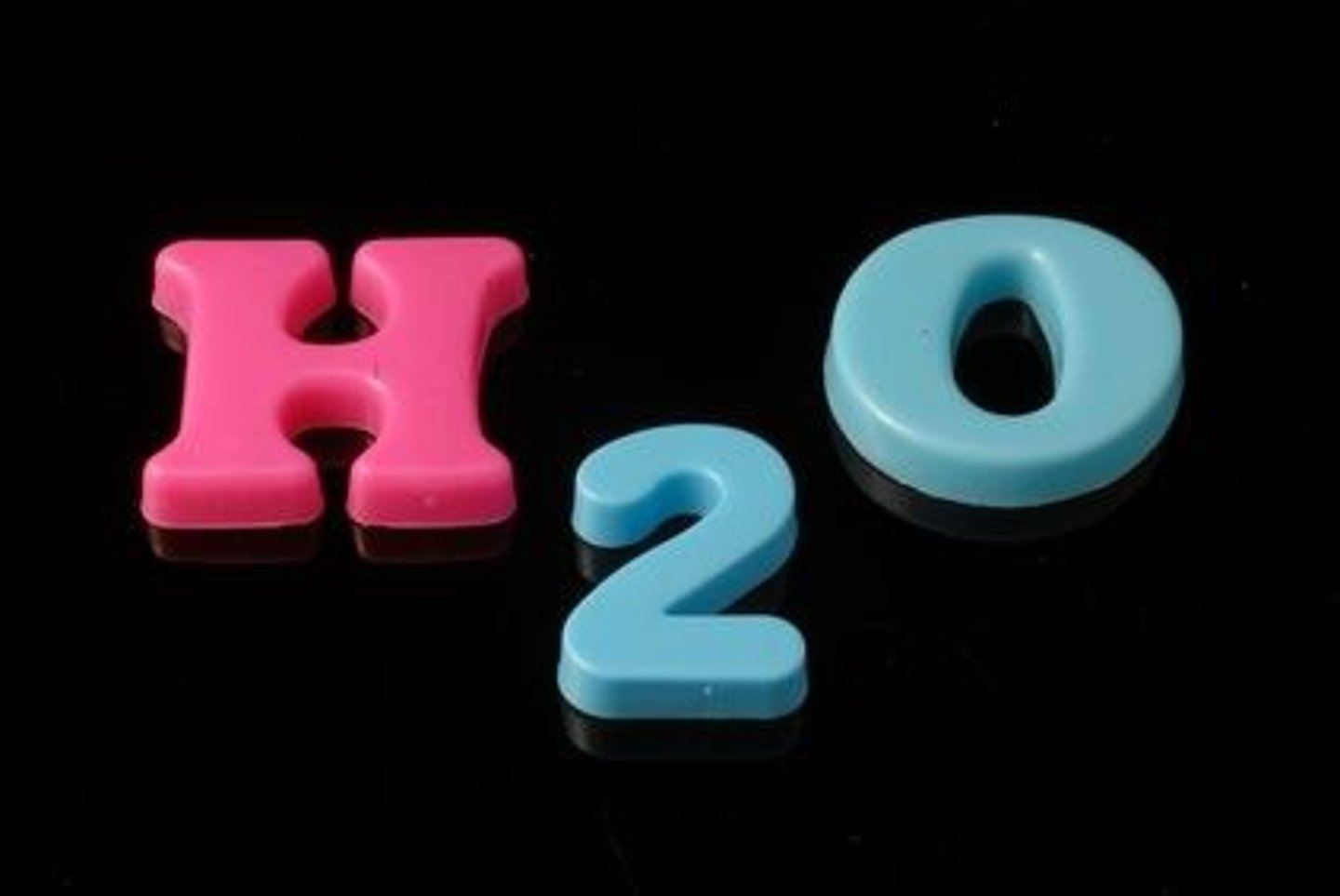
Chemical Symbol
One or two letter abbreviation for an element

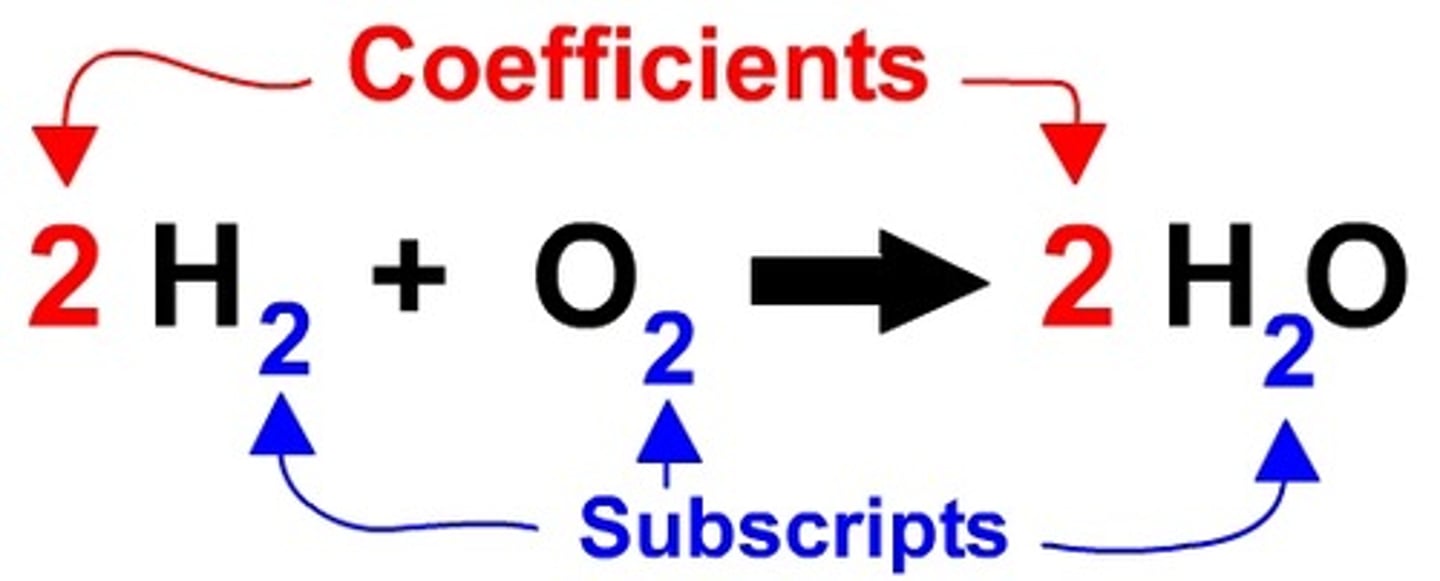
Subscript
Number to the lower right of a chemical symbol; indicates how many atoms of that element are in the compound
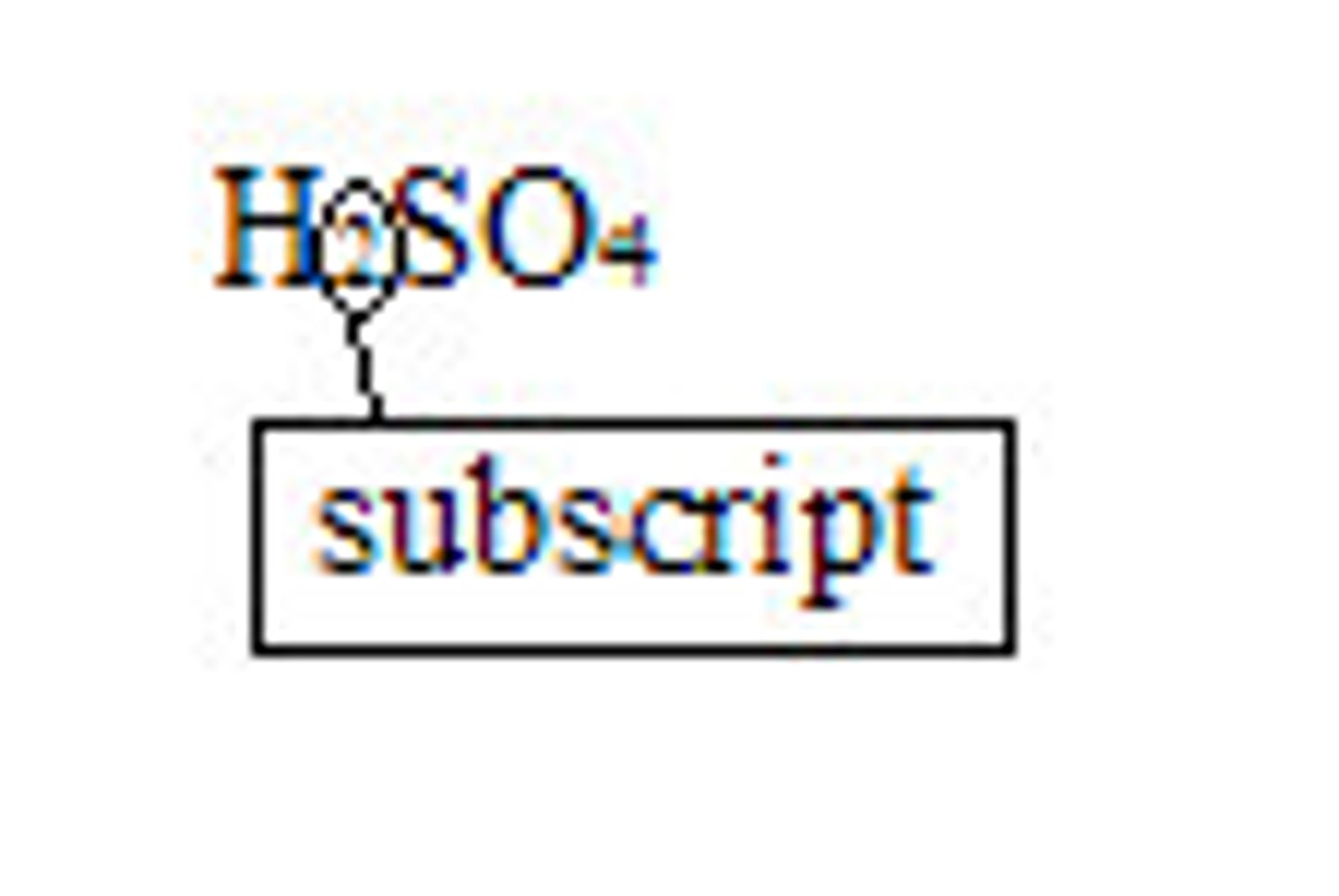
Chemical Equation
Use of chemical symbols and formulas to indicate a chemical reaction
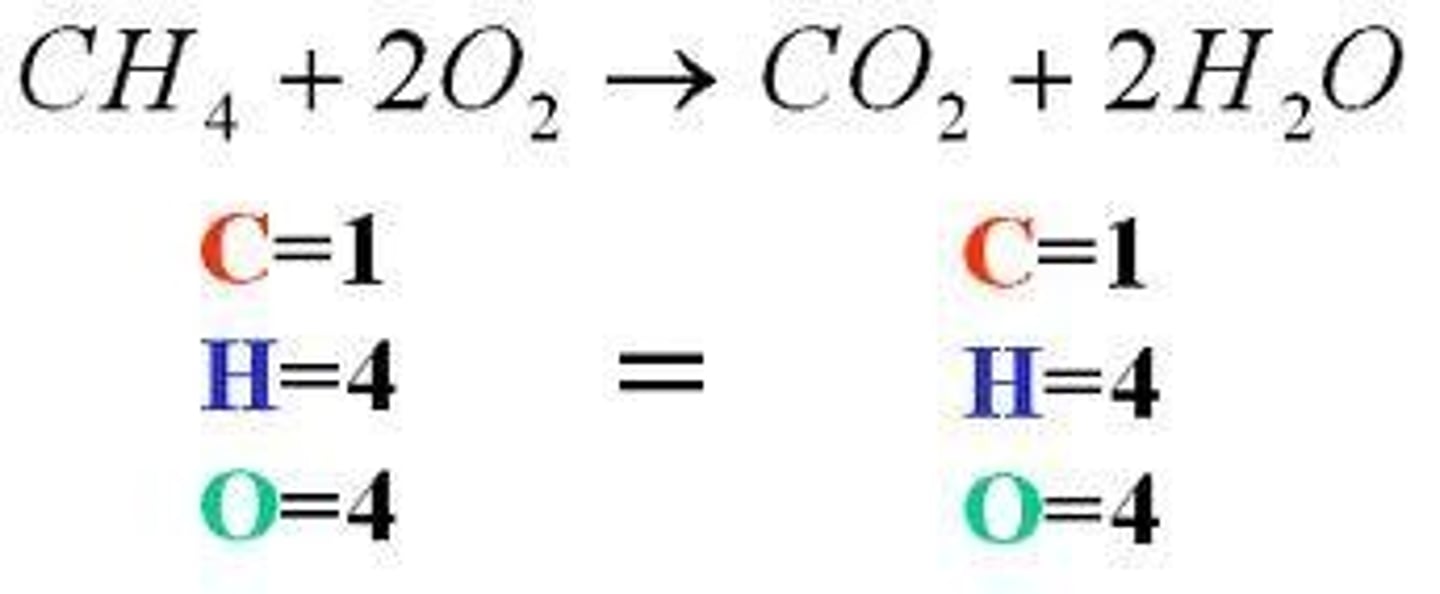
Reactants
Starting materials in a chemical reaction
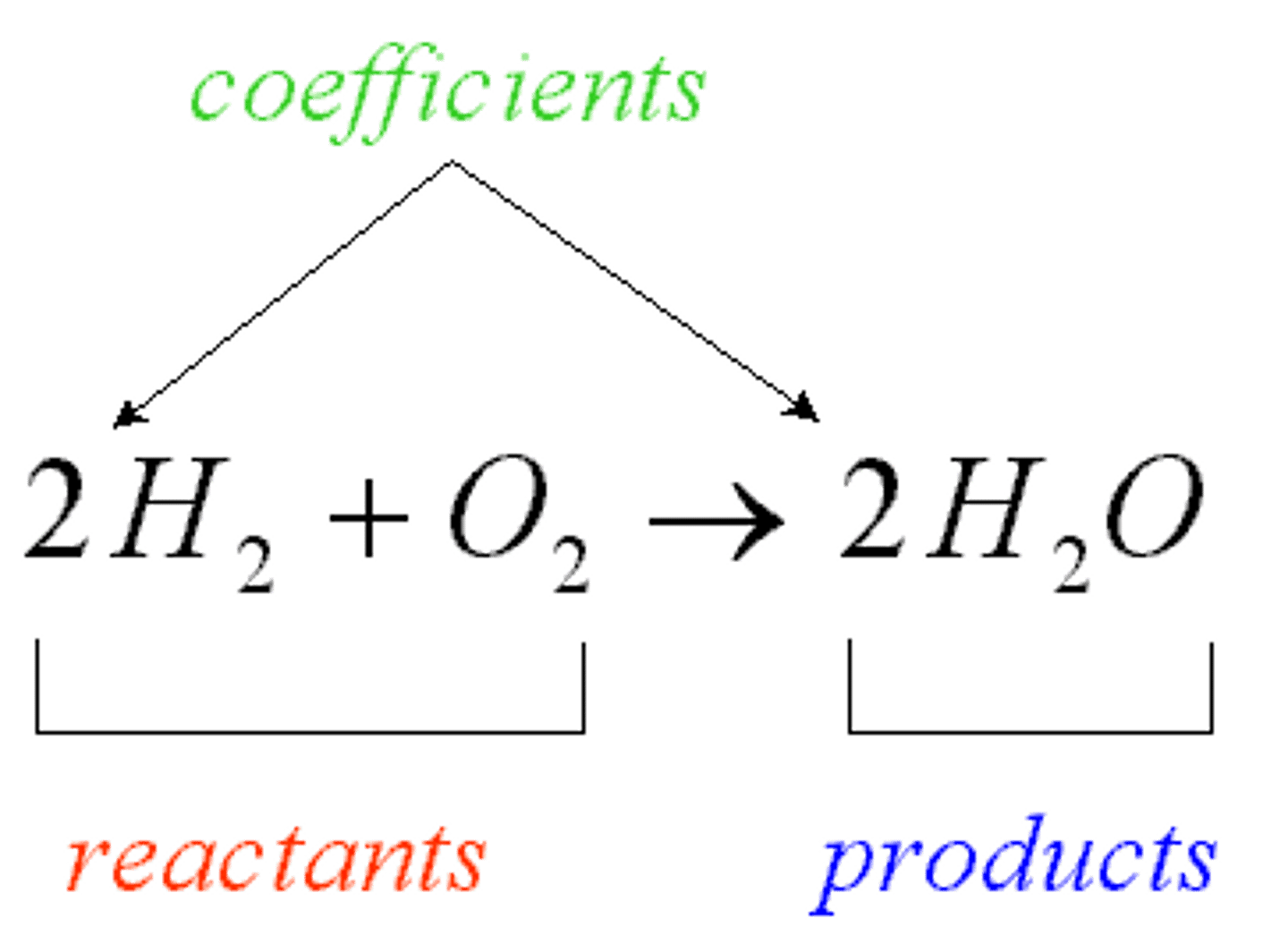
Products
Ending materials in a chemical reaction
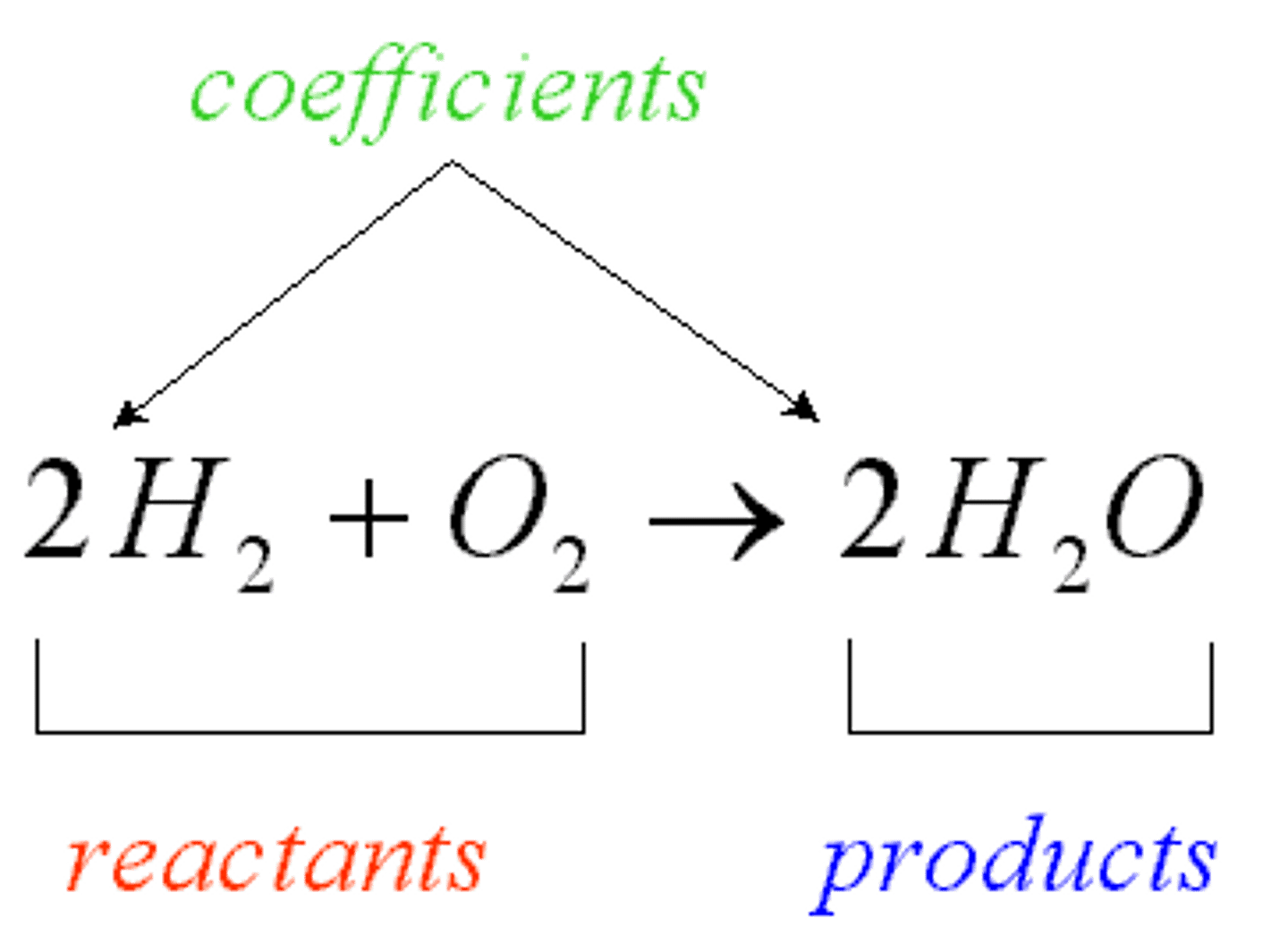
Coefficient
Number in front of a chemical formula indicating how many of that atom or molecule are needed in an equation
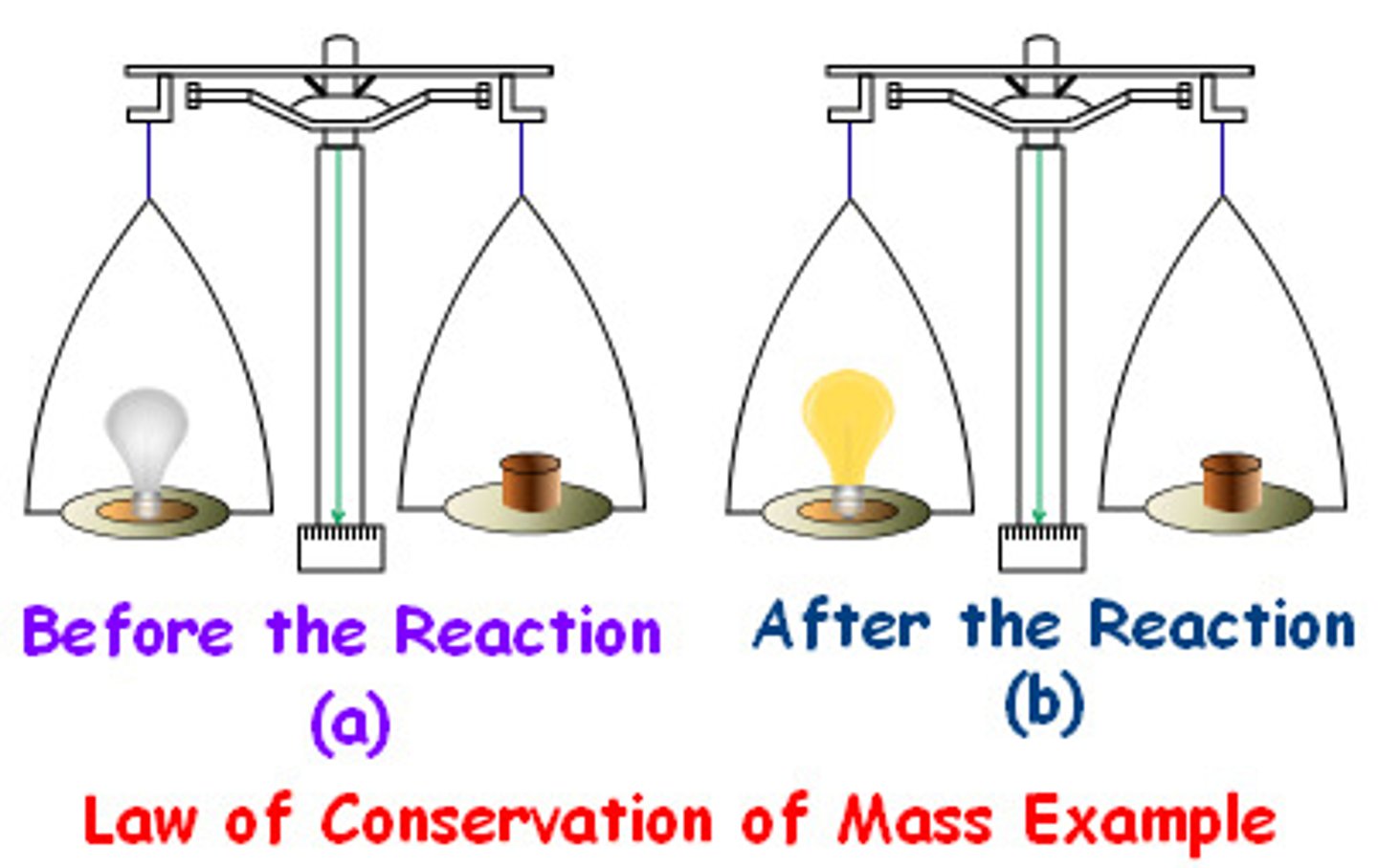
Law of Conservation of Mass
The mass of the reactants is equal to the mass of the products
synthesis reaction
a reaction in which two or more substances combine to form a new compound

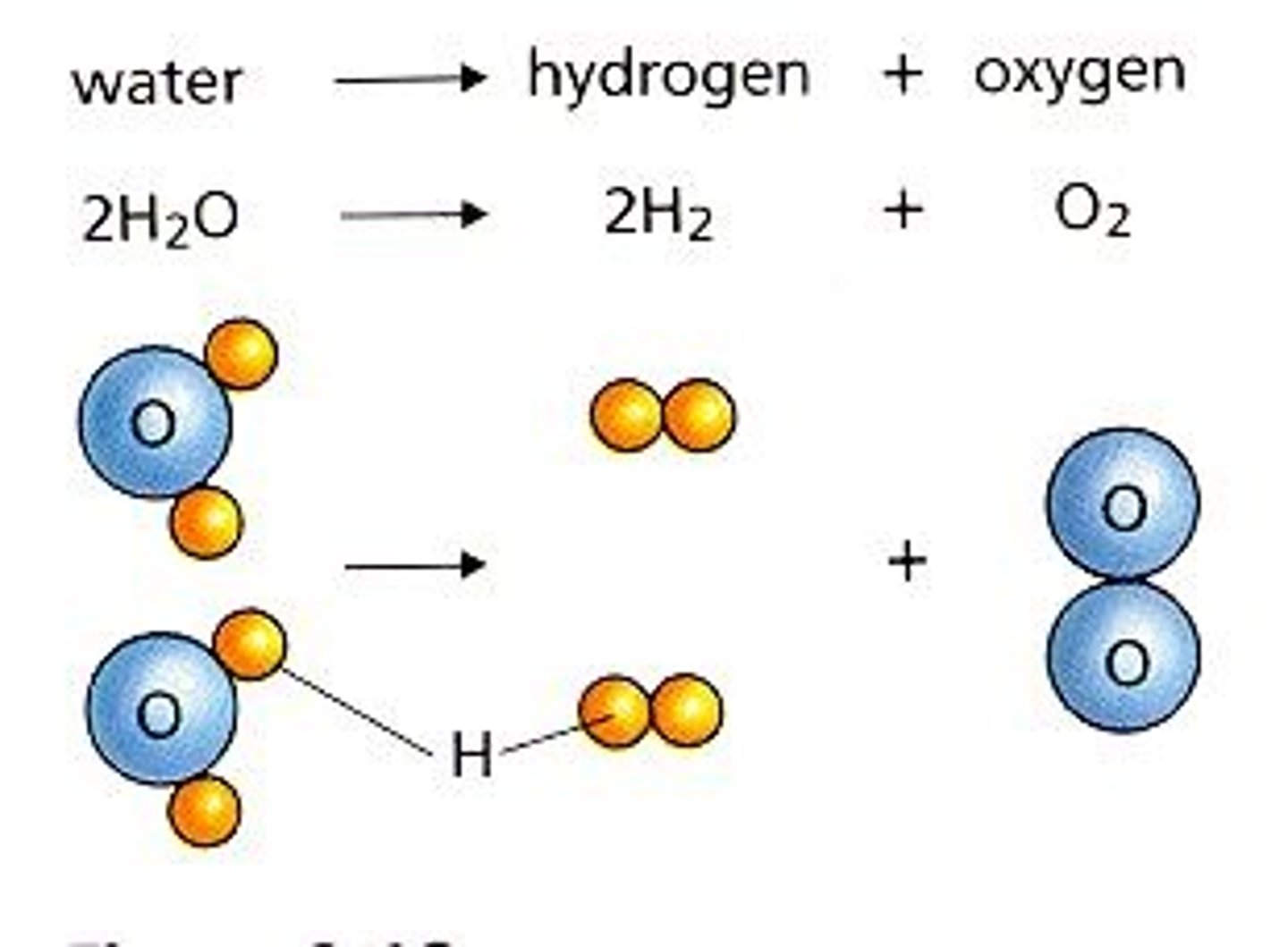
decomposition reaction
a reaction in which a single compound breaks down to form two or more simpler substances
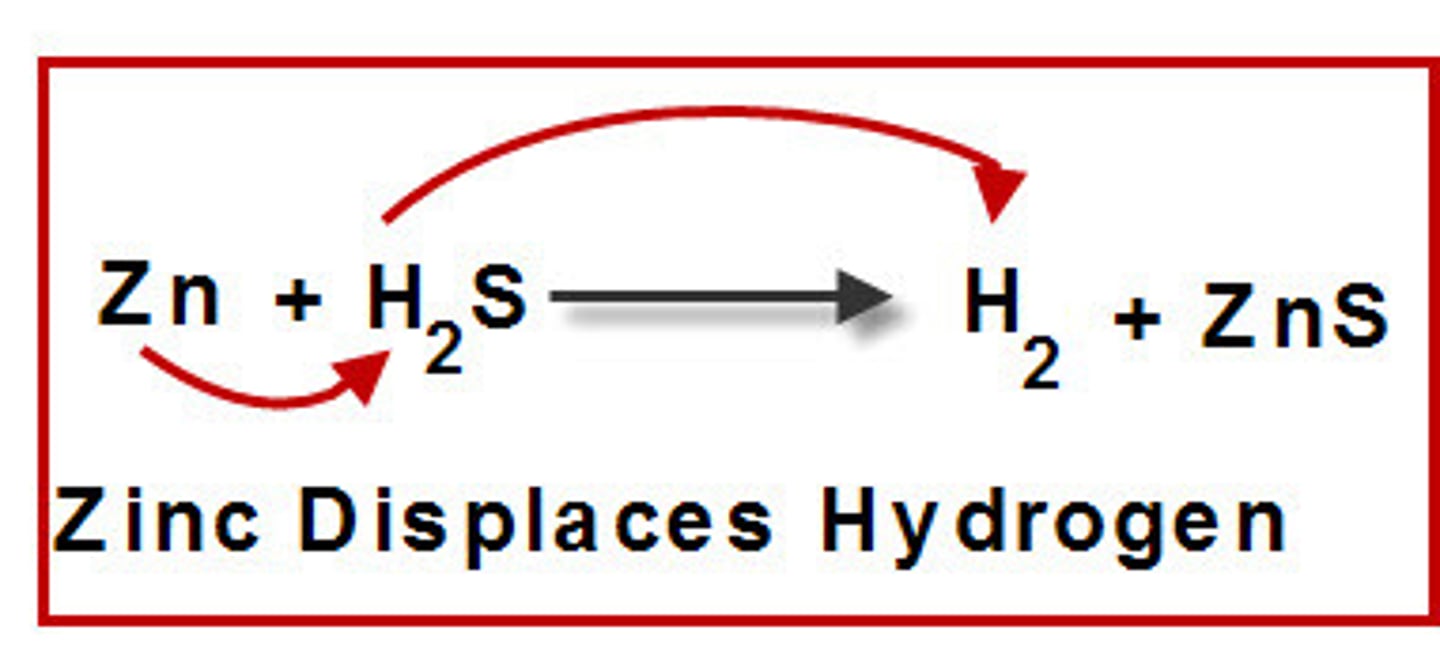
Single Displacement/Replacement reaction
one element replaces a similar element in a compound
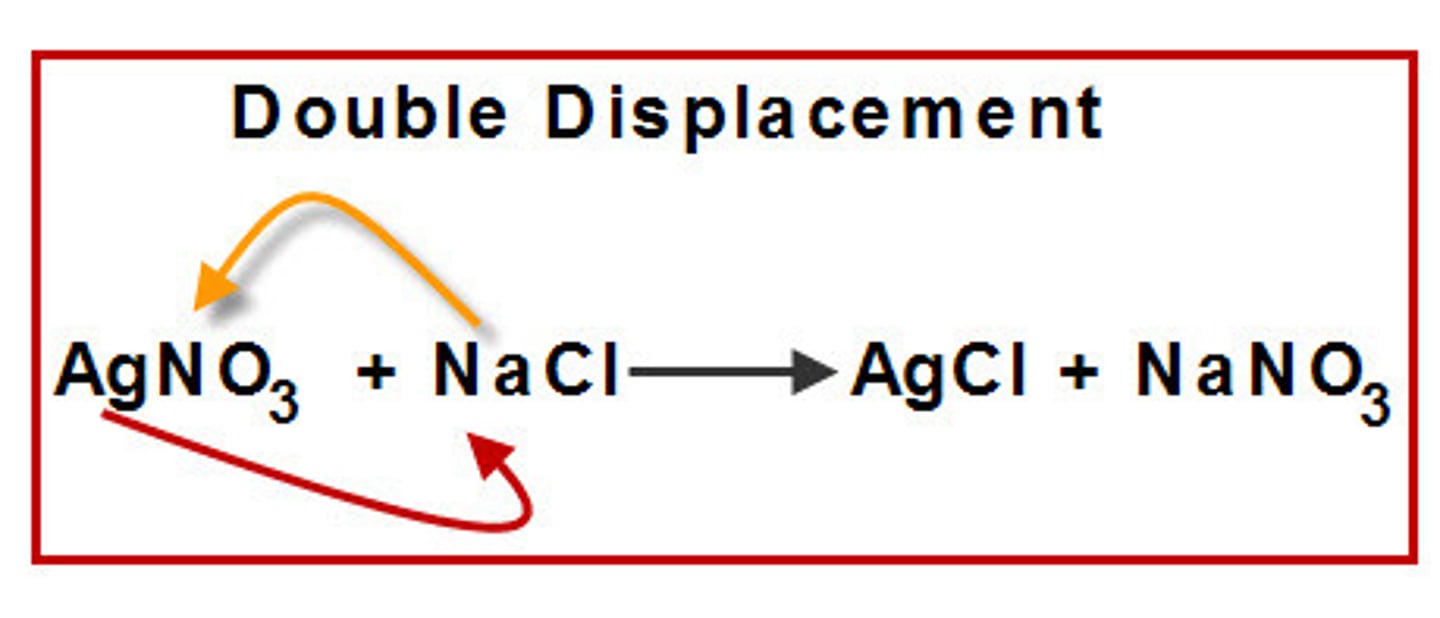
Double Displacement/Replacement reaction
a chemical reaction where two compounds react to form two or more new compounds by switching ionic partners (positive & negative ions switch)
combustion reaction
a chemical reaction that occurs when a substance reacts with oxygen, releasing energy in the form of heat and light