Final Exam- Organic Chemistry 1
1/65
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
66 Terms
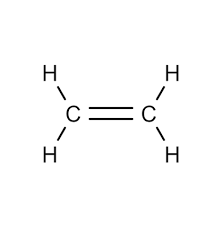
Alkenes
Alkynes
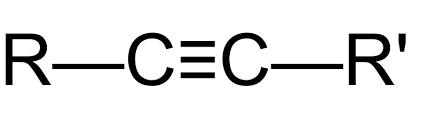
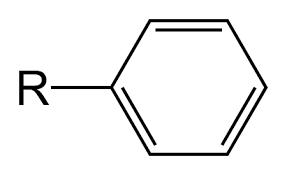
Phenyl
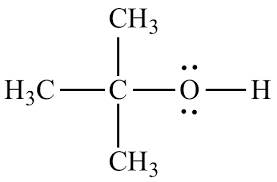
Alcohol
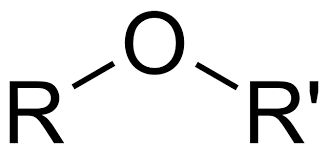
Ether
Nitrile
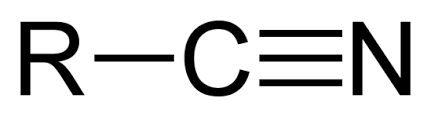
Amine

Thiol
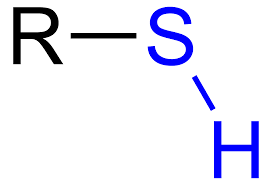
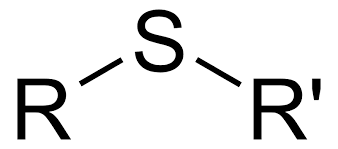
Sulfide
Aldehyde
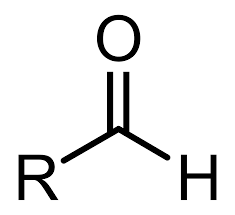
Ketone
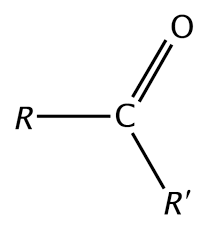
Ester
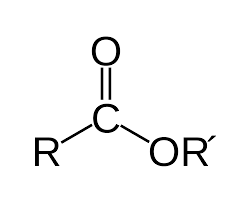
Amide
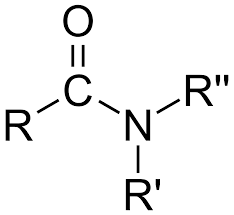
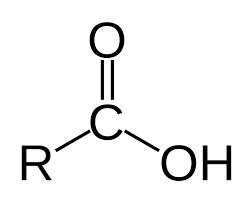
Carboxylic Acid
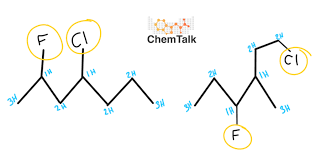
Constitutional isomers
Molecules with the same molecular formula but different structural arrangements.
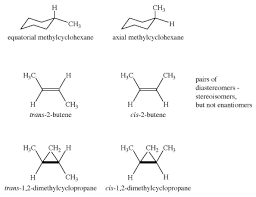
Stereoisomers
Molecules that have the same molecular formula and connectivity but differ in the spatial arrangement of atoms.
Achiral molecules
are molecules that do not have chiral centers and are superimposable on their mirror images.
Chiral Molecules
are molecules that have chiral centers, meaning they cannot be superimposed on their mirror images.
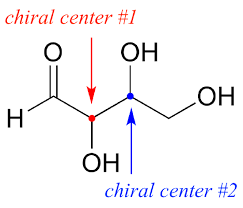
Chiral Center
A carbon atom bonded to four different substituents, resulting in non-superimposable mirror images.
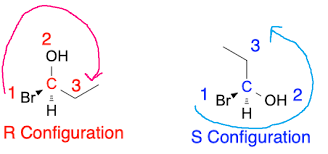
R Configuration
Refers to the specific spatial arrangement of atoms around a chiral center that follows the Cahn-Ingold-Prelog priority rules, resulting in a counterclockwise orientation.
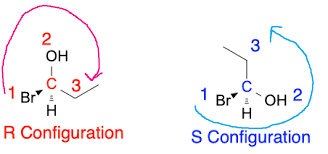
S Configuration
Refers to the spatial arrangement of atoms around a chiral center that follows the Cahn-Ingold-Prelog priority rules, resulting in a clockwise orientation.
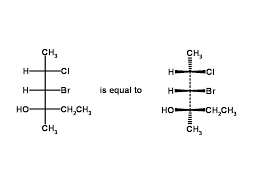
Fischer Projections
A two-dimensional representation of a three-dimensional organic molecule, where the horizontal lines represent bonds that project out of the plane, and vertical lines represent bonds that project into the plane.
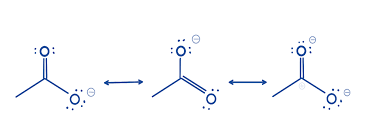
Resonance Structures
Different ways to draw a molecule that show the delocalization of electrons, maintaining the same arrangement of atoms but with varying electron positions.
Enantiomers
Stereoisomers that are non-superimposable mirror images of each other, possessing chiral centers.
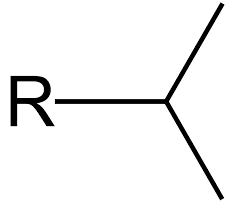
Isopropyl
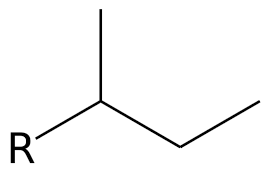
Sec-butyl
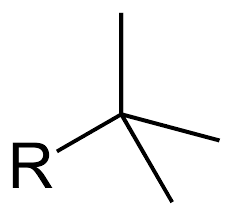
Tert-butyl
Halogenation of Alkanes
A chemical reaction where alkanes react with halogens to form haloalkanes, typically involving free radicals. This process often requires heat or light to initiate.
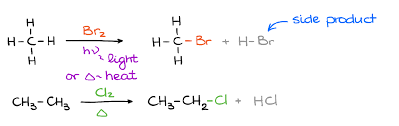
Combustion of Alkanes
A chemical reaction in which alkanes react with oxygen to produce carbon dioxide and water, releasing energy in the form of heat and light.
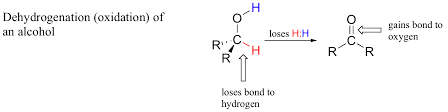
Oxidation
A chemical reaction where a substance loses electrons, often involving the addition of oxygen or the removal of hydrogen. Oxidation is a key process in combustion and respiration.
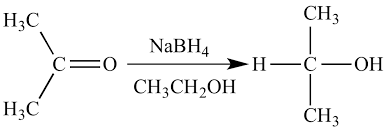
Reduction
A chemical reaction where a substance gains electrons, often involving the addition of hydrogen or the removal of oxygen. Reduction is the opposite of oxidation and plays a crucial role in various biological and chemical processes.
Chlorination of Alkanes
A chemical reaction in which alkanes react with chlorine, typically under ultraviolet light, to form alkyl chlorides and hydrochloric acid. This process is important in organic chemistry for the synthesis of chlorinated hydrocarbons.
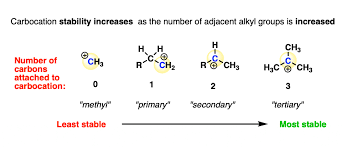
Carbocation stability
refers to the relative stability of a carbocation, which is a positively charged carbon species. Stability is influenced by factors such as hybridization and the presence of electron-donating groups.
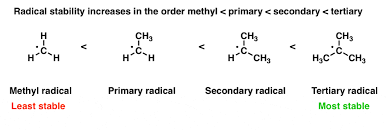
Radical Stability
Refers to the relative stability of free radicals, which are species with unpaired electrons. Stability is influenced by factors such as the radical's hybridization and the presence of electron-donating groups.
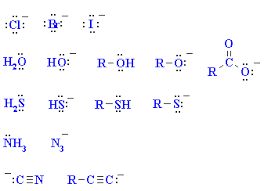
Nucleophiles
are chemical species that donate an electron pair to form a chemical bond in a reaction. They are typically Lewis bases and play a vital role in nucleophilic substitution and addition reactions.
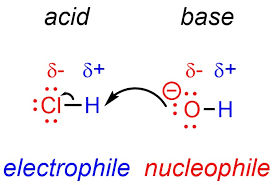
Electrophile
is a chemical species that accepts an electron pair to form a bond in a reaction. They are usually Lewis acids and are essential in electrophilic substitution and addition reactions.
Substitution Reactions
Involves the replacement of one atom or group in a molecule with another. These reactions can be classified as nucleophilic or electrophilic, depending on the nature of the reacting species.
Features:
1) Charge of Nu
2) The leaving group
3) identity of the Nu
4) The solvent
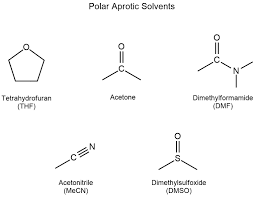
Polar Aprotic Solvents
are solvents that do not have acidic hydrogen atoms but can solvate cations effectively. They play a significant role in facilitating nucleophilic substitution reactions by stabilizing the nucleophile without forming strong hydrogen bonds.
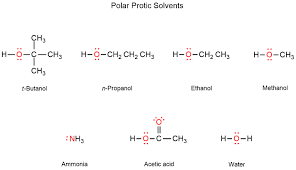
Polar Protic Solvents
are solvents that contain acidic hydrogen atoms, which can form hydrogen bonds with solutes. They stabilize nucleophiles through hydrogen bonding, thereby influencing reaction mechanisms such as nucleophilic substitutions.
Substitution of alcohols
is a chemical reaction where an alcohol group is replaced by another atom or group, often involving nucleophilic substitutes.
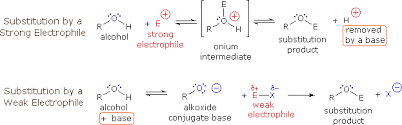
3 Alternative reagents for the formation of alkyl halides from alcohols
include hydrogen halides, phosphorus tribromide (PBr3), and thionyl chloride (SOCl2), (-OTs), (HBr), (HI), (HCl).These reagents effectively convert alcohols into alkyl halides through different mechanisms, facilitating nucleophilic substitution reactions.
Regiochemistry
is the study of the distribution of electrons across the molecular structure, determining the regions where reagents or groups will add or react. It plays a crucial role in defining the products of chemical reactions.
Tetra-Substituted Alkenes
are alkenes that have four substituents attached to the double bond, leading to greater stability and unique chemical properties compared to less substituted alkenes.
Tri-Substituted Alkenes
are alkenes that have three substituents attached to the double bond. They are more stable than di-substituted alkenes but less stable than tetra-substituted alkenes.
Di-Substituted Alkenes
are alkenes with two substituents attached to the double bond. They exhibit increased stability compared to mono-substituted alkenes but are less stable than tri-substituted alkenes.
Mono-Substituted Alkenes
are alkenes that have one substituent attached to the double bond. They are the least stable among substituted alkenes.
Zaitsev’s Rule
is a guideline in organic chemistry that states that the more substituted alkene product will be favored in elimination reactions.
Elimination Reactions
are chemical reactions where a pair of atoms or groups is removed from a molecule, often forming a double bond in alkenes.
Sn1 Reactions
are a type of nucleophilic substitution reaction that involves a two-step mechanism, where the formation of a carbocation intermediate is key.
Sn1 Reactions
reactions with tertiary carbocations, polar protic solvents, and weak nucleophiles. They are also favored when the substrate can form a relatively stable carbocation intermediate.
Sn2 Reactions
are a type of nucleophilic substitution reaction that occurs in one concerted step. They typically involve primary or secondary alkyl halides with strong nucleophiles.
Sn2 Reactions
unhindered substrates (methyl or primary), strong nucleophiles, and polar aprotic solvents. They also require a good leaving group.
E2 Reactions
are a type of elimination reaction that occurs in one step, typically involving strong bases and substrates that can stabilize a double bond.
E2 Reactions
strong bases, secondary and tertiary alkyl halides, and high concentrations of both reactants. They also favor the formation of trans-double bonds and Zaitsev products. Reactions are favored in polar aprotic solvents, such as acetone, acetonitrile, and dimethylformamide (DMF)
E1 Reactions
are a type of elimination reaction that occurs in two steps, typically involving weak bases and tertiary substrates, leading to the formation of a carbocation intermediate.
E1 Reactions
Polar protic solvents like water (H₂O) and alcohols (ROH) are generally favored. Weak bases, a substrate that forms a stable carbocation, and an ionizing solvent. These conditions promote the formation of a carbocation intermediate
Markovnikovs Rule
States that in the addition of HX to an alkene, the hydrogen atom attaches to the carbon with more hydrogen atoms, giving rise to the more stable carbocation.
Anti-Markovnikovs Rule
States that in the addition of HX to an alkene, the hydrogen atom attaches to the carbon with fewer hydrogen atoms, leading to a less stable carbocation.
Sym Addition
A type of addition reaction where two substituents are added to the same side of a double bond, resulting in a syn configuration.
Anti Addition
A type of addition reaction where two substituents are added to opposite sides of a double bond, resulting in an anti configuration.
Z Conformation
isomerism refers to a configuration of alkenes where the highest priority groups are on opposite sides of the double bond.
E Conformation
A configuration around a double bond where the highest priority substituents on each carbon are on opposite sides, resulting in a trans-like arrangement.