Energy Changes and Rates of Reaction - Thermodynamics
1/58
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
59 Terms
thermochemistry
the study of energy changes that accompany physical and chemical changes in matter
three types of changes that occur in matter
physical (changing state), chemical, nuclear
all are accompanied by a change in en
fission
large atom splits into two or more smaller atoms
way stronger than other en sources
used in nuclear power plants
fusion
joins two or more lighter atoms to make a larger and heavier one
in development
way stronger than fission
energy
the ability to do work
three main ways to store en
kin en
poten en
nucl en
kin en
the energy of an object due to its motion (translation, rotation, vibration)
poten en
the energy of a body or system due to its position or composition – found in chemical bonds (released when new bonds form)
nucl en
stored in the nucleus as energy needed to hold the nucleus together
therm en
total potential and kinetic energy of a substance
often considered a form of kinetic energy due to its connection to the motion of molecules.
heat
the transfer of thermal energy from a warm object to a cooler object
temp
the average kinetic energy of the particles in a sample, measured in °C or K
energy flows between substances because of their ________________ in temperature
difference
law of conservation of energy pts (4)
The total energy of the universe is constant (1st Law of Thermodynamics)
Energy can neither be created nor destroyed
Energy can be transferred from one substance to another
Can be converted into various forms
chemical system
a set of reactants and products under study, usually represented by a chemical equation
surroundings
all matter around the system that is capable of absorbing or releasing thermal energy
change in en in universe =
change in en system + change in en surrounding = 0
any change in the system is accompanied by an _____________________ change in the surroundings
equal (magnitude) nd opposite (sign)
three types of systems
open system
closed
isolated
open system
A system where both matter and energy can move in and out (Example: Open mug)
closed system
A system where energy can move in and out, but not matter (Example: Coffee cup with lid)
isolated system
An ideal system, where neither energy or matter can move in or out (Example: insulated coffee cup with lid)
total en of a system =
PE + KE of all species in the system
kin en
moving e
vibration, rotation and translation of atoms and molecules
poten en
nuclear poten en of protons and neutrons
bond en (stored en)
intermolecular forces
exothermic rxns
release therm en
heat (q) flows from the system to the surroundings, usually causing an increase in the temperature of the surroundings
q has a negative value (q < 0); i.e. losing heat from the system
endothermic rxns
absorb thermal energy
heat (q) flows from the surroundings into the system, usually causing a decrease in the temperature of the surroundings
q has a positive value (q > 0); i.e. adding heat to the system
calorimetry
technological process of measuring energy changes in a chemical system
heat of a reaction can be measured in this isolated environment
specific heat capacity
denoted as “c'“
units : J/g°C OR J/g°K
the amount of energy required to raise the temperature of 1 g of a substance by 1°C or one K; depends on type of substance and state of substance
amount of heat transferred formula
q= mcΔT
The amount of heat transferred (q) (can be neg or pos), in Joules (J), depends on:
mass of sample (m) measured in grams
temperature change (ΔT) measured in °C or K (can be neg or pos)
specific heat capacity (c) measured in J/g•°C (or J/g•K)
water specific heat capacity
4.18 J/g*C
q (heat)
mag of q tells you how much en is involved
sign of q tells you whether the system lost or gained en from the surroundings
q (cont’d)
q (system) = -q (surroundings)
density
mass (g) per volume (mL)
2nd law of thermodynamics
when 2 objects are in thermal contact, heat is always transferred from the object at a higher temperature to the object at a lower temperature, until the 2 objects are at the same temperature (= “thermal equilibrium”)
aq solutions use the heat capacity of
water (4.18)
energy is a __________________
state function → it is INDEPENDENT of how the change happens and depends only on the states of the initial reactants and the final products in a system
molar enthalpy
energy released or absorbed per mole of a substance
why are multiple bonds more energetic than single bonds
The higher the bond enthalpy the more energy is required to break that bond and hence the stronger the bond.
hess’s law
states that the enthalpy change of a process is the same whether the process takes place in one step or in a series of steps
hess’s law formula
H( r ) = H1 + H2 + H3 ….
why is hess’s law helpful?
calculating enthalpies of reactions that are difficult to study in the lab
difficult because chemicals are expensive, dangerous, or unpredictable
hess’s law 1
When a chemical reaction is reversed, the sign of its ∆H changes
hess’s law 2
When a chemical reaction is multiplied by a coefficient, the same is true of its enthalpy
premise of bond energies
all reactions involve bond breaking and bond making as the atoms recombine
bond breaking
energy is always required to break a bond. Bond breaking is endothermic
bond making
energy is always released when a bond is formed. Bond making is always exothermic
formula for bond energy
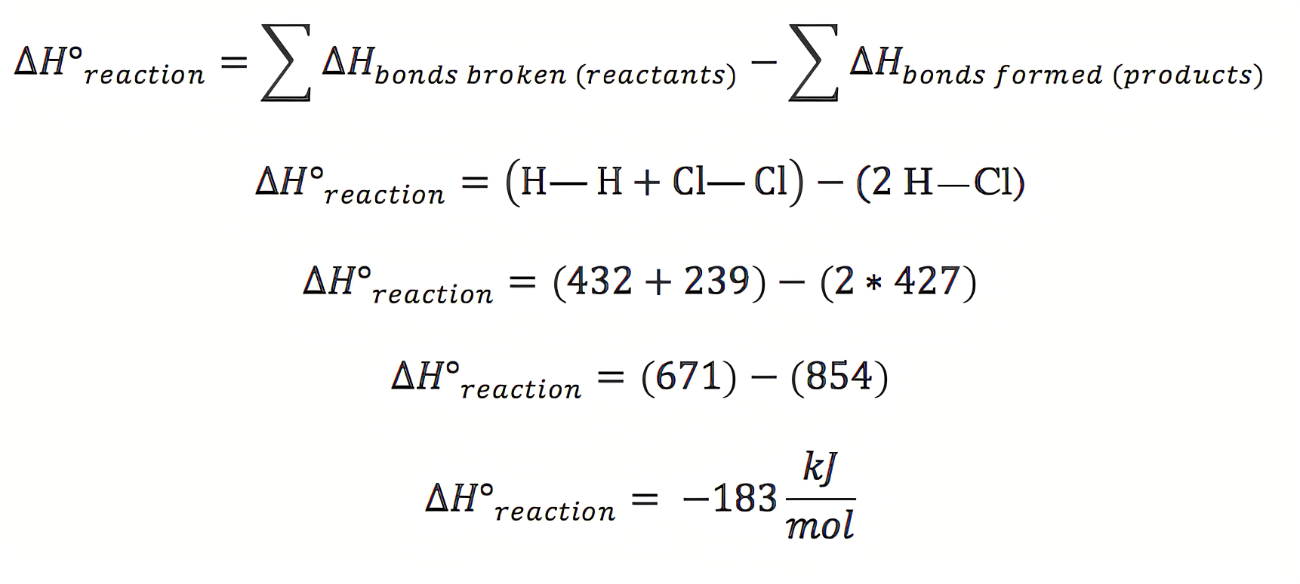
potential en diagram w bond en
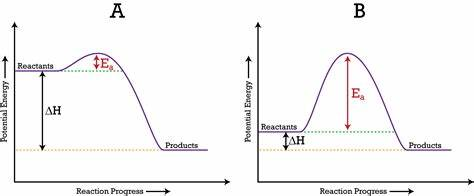
exothermic potential en diagram (with bond en)
less energy is needed to break the bonds than is released when forming the new compounds
endothermic potential en diagram (with bond en)
more energy is required to break the bonds than is released when forming new compounds
formula for standard formation
products minus reactants
bond dissociation energy
quantity of energy required to break a chemical bond
Bond dissociation energies are ____________ values
positive
reported energies are an _____________ bond en
avg
as the # of bonds increases, the length of the bond ____________
decreases
en must be _________ to break bonds
added (endo, pos)
en is _____________ when forming bonds
released (exo, neg)
units for rate of rxn
mol / L * s
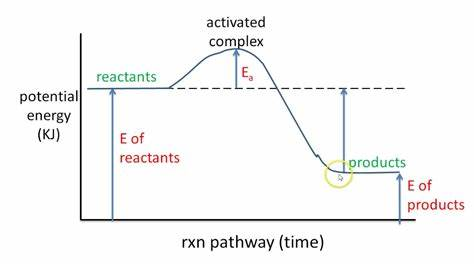
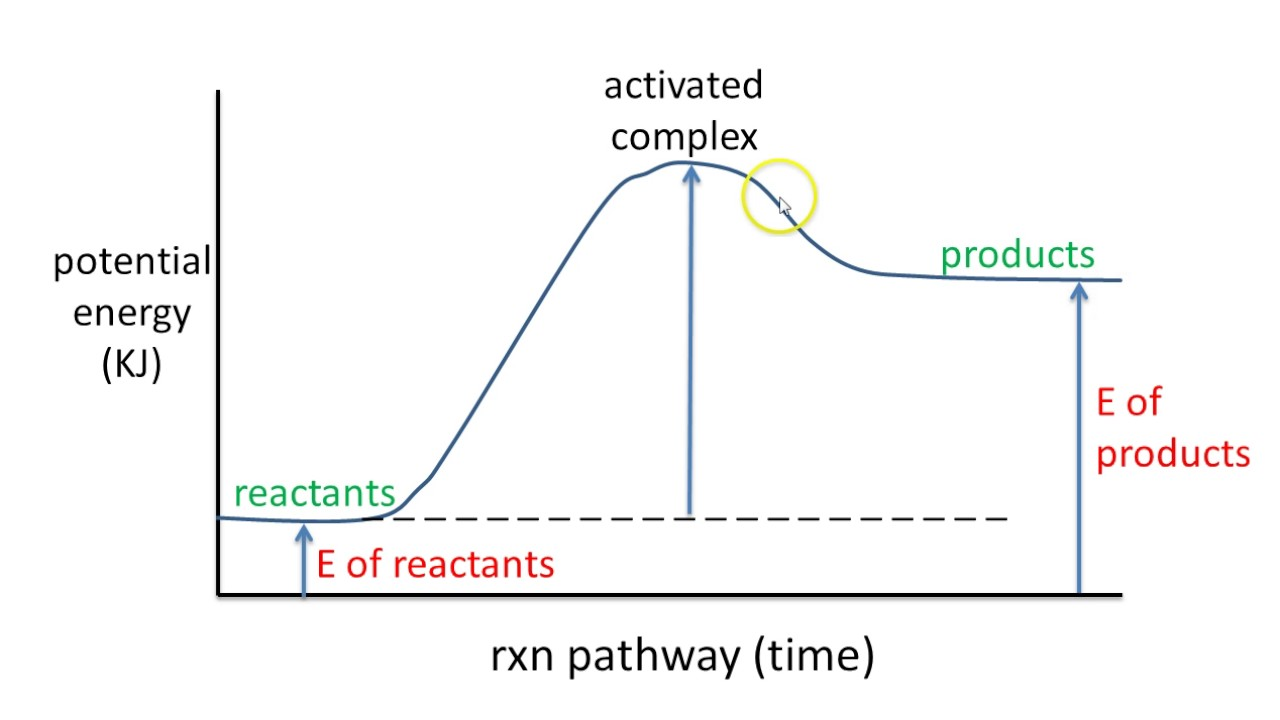
in chemical rxns, both products and reactants have
potential en