antibiotics and antimicrobial stewardship
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
41 Terms
antibiotic
a naturally occurring agent produced by a microbe that inhibits or kills another.
Now - any antibacterial agent. Either synthetic, semi-synthetic or naturally occurring drug
anti-virals
target viruses
anti-fungals
treat fungal infections
antimicrobial
any drug that inhibits or kills a bacterium, virus, fungus etc.
selective toxicity
agent being used should inhibit or kill the intended pathogen without seriously harming the host
selectively attack targets that are in bacteria cells but not in human cells
example: cell wall or protein building/DNA copying machinery
antibiotic therapy treat of infection
smart smart: Empiric therapy: Treatment of an infection before specific culture information has been reported or obtained
then focus: targeted therapy: treatment focused to known pathogen and susceptibility
antibiotic therapy prevention of infection
prophylactic therapy: administration of antibiotics to prevent an infection
classification of antibiotics
antibiotic family
bacterial or bacteriostatic
narrow or broad-spectrum
mechanism of action i.e. cell-wall agents, inhibitors of protein synthesis
antibiotic family
bacteriostatic - antimicrobial activity
inhibit growth of susceptible, rather than killing them immediately
will eventually lead to bacterial death
immune defenses important for clearing pathogen
bactericidal - antimicrobial activity
kills susceptible bacteria
important to use if immunodeficient or for certain difficult infections e.g. meningitis, endocarditis and infection
narrow antibiotic spectrum
active against specific organisms or types of organism e.g. penicillin G active against GPC only
minimal disruption of normal flora
not suitable for blind therapy
broad antibiotic spectrum
active against many bacteria e.g. piperacillin-tazobactam, effective against some GPC, some GNB including pseudomonas and anaerobic bacteria
polymicrobial/unknown aetiology
superinfection e.g. clostridium difficile
importance of the spectrum of activity
incorrect method may not kill the bacteria - infection may progress, may increase development of resistance, may kill non-pathogenic bacteria causing change in normal flora and risk of superinfection
C. difficile infection
clostridioides difficile infection
spore forming bacterium that lives in the bowel
infection of the colon
symptoms: watery diarrhea, fever, nausea, abdominal pain
in severe cases: can lead to toxic megacolon or sepsis and be life threatening
10 times more likely to get it after antibiotic use
can occur as antibiotic started up to three months after it is completed
healthy microbiome protects from D. difficile - disrupted by antibiotic use.
groups of bacteria in terms of antibiotic treatment
Gram positive
gram negative
anaerobes
atypical
Gram + VE
staphylococci
streptococci
enterococci
clostridia
corynebacterial
listeria
bacillus
Gram - VE
E coli, klebsiella
proteus, salmonella, shigella
pseudomonas
haemophilus
helicobacter, campylobacter
legionella
Neisseria meningitidis
Neisseria gonorrhoea
Moraxella catarrhalis
Acinetobacter
Bacteroides
atypical bacteria and mycobacteria
atypical bacteria
chlamydia - intracellular pathogen
mycoplasma and ureaplasma - small bacteria that lack a cell wall
mycobacteria
M. tuberculosis, M. avium complex, M. leprae
Gram + VE agents
narrow spectrum
penicillin V/G, flucloxacillin
Fusidic acid, Rifampicin
clindamycin
daptomycin
vancomycin, teicoplanin
linezolid
Broad spectrum
cefalexin, co-amoxiclav, cefuroxime, ceftriaxone
minocycline, doxycycline, tetracycline, tigecycline
erythromycin. clarithromycin, azithromycin
trimethoprim
gram -VE agents
narrow spectrum
colistin
broad spectrum
gentamicin, tobramycin, amikacin (aminoglycosides)
ciprofloxacin, moxifloxacin (quinolones)
minocycline, doxycycline, tetracycline (tetracyclines)
cefalexin, cefuroxime, co-amoxiclav (beta-lactams)
trimethoprim, nitrofurantoin
anti anaerobe agents
metronidazole
clindamycin
co-amoxiclav
piperacillin-tazobactam
meropenem
anti-atypical agents
tetracyclines: tetracycline, minocycline, doxycycline, oxytetracycline, tigecycline
macrolides: erythromycin, clarithromycin, azithromycin
quinolones: ciprofloxacin, moxifloxacin, levofloxacin
modes of action
breach their walls - cidal
cell wall active agents e.g. beta lactams, glycopeptides
hack into their information center
antimetabolites (trimethoprim)
inhibit RNA synthesis (rifampicin) or DNA replication (ciprofloxacin)
disrupt their factories
inhibitors of protein synthesis e.g. gentamicin
susceptibility testing oorgansim
determine antibiotic susceptibility profile of an infecting organism
minimum inhibitory concentration (MIC)
susceptibility profile can aid bacterial identification and epidemiology
susceptibility testing patient
predicts patient response
empiric therapy often begins before this result is available so epidemiological data supports empiric therapy
results are important
if patient fails to respond to therapy allow to change to more appropriate agent e.g. with respect to cost, sensitivity, effectiveness or side effects
susceptibility testing methods
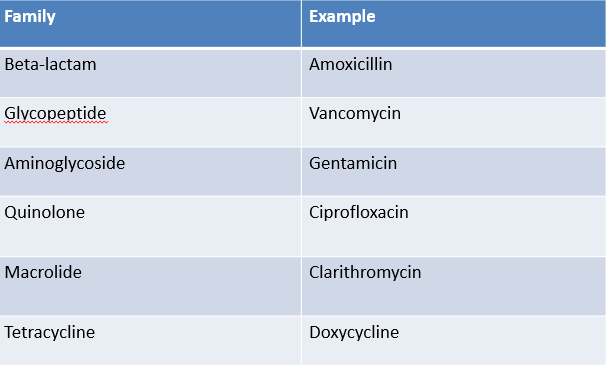
disk diffusion - result is that organism is susceptible or resistant
minimum inhibitory concentration (MIC) - result is a value that indicates how susceptible/resistant the organism is e.g. broth microdilution or E-test
disk diffusion
inoculate surface of agar plate with test organism
apply cellulose discs with standard amount of antibiotic on an agar plate
antibiotic diffuses into agar and zone sizes reflect susceptibility or resistance
easy to do, cheap, test 4-6 agents at one time
not quantitative, only semi quantitative at best
many different standard methods e.g. BSAC, Stokes, CLSI
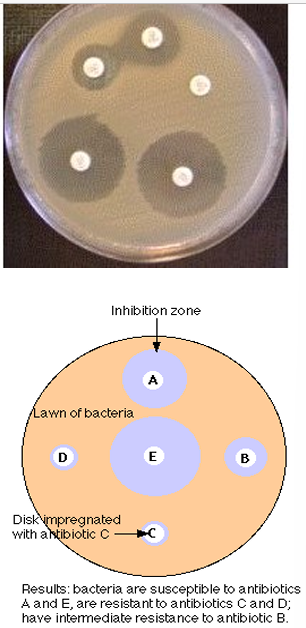
minimum inhibitory concentration
the lowest concentration of an antibiotic required to inhibit growth of a bacterium
determined by testing the ability of the test organism to grow in the presence of the drug in a series of concentrations in either broth or on agar
selective organisms e.g. strep pneumoniae and penicillin
can detect changes in susceptibility
broth microdilution
fixed concentration of bacteria added to tubes/microwell plates
grow in the presence of increasing concentration of antibiotic
examine growth after 24h
at what concentration does it inhibit growth
MIC is the lowest concentration that inhibits growth
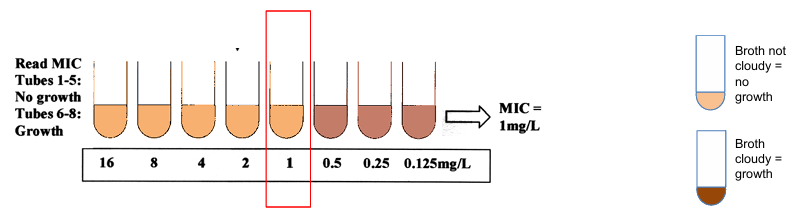
E test
an agar based quantitative susceptibility test
diffusion method that enables MIC values to be estimated directly
an extended series of two fold dilutions of an initially high standard concentration of antibiotic
antibiotic concentrations are distributed linearly along a special carrier strip
MIC is the concentration corresponding to the point of intersection of the eclipse zone as shown
ideals in antibiotics
selective toxicity - toxic to the target pathogen but not the host
bactericidal vs. bacteriostatic (host defenses)
favorable pharmacokinetics - reaches target site in the body with effective concentration (good bioavailability)
spectrum of activity - broad vs. narrow
lack of side effects - effective to toxic dose ratio
little resistance development - can withstand bacterial resistance
antimicrobial stewardship
key strategy to overcome resistance
involves the careful and responsible management of antimicrobial use
improves patient outcomes - improve infection cure rates, reduce surgical infection rates, reduce morbidity and mortality
improve patient safety - reduce antimicrobial consumption without increasing mortality or infection related re admissions
reduce resistance - restricting relevant agents can reduce colonization or infection with resistant bacteria
reduce healthcare costs without compromising patient care
factors affecting antibiotic choice
concentrations of antibiotics achievable may differ at different sites
bacteria vs. viruses
gram positive or negative
accompanying treatment
how effectively or fast the drug are absorbed, distributed and metabolized
antibiotics and their most likely infection
anaerobes - mouth, teeth, throat, sinuses and lower bowel → abscesses, dental infection, peritonitis, appendicitis
atypical - chest and Genito-urinary → pneumonia, urethritis, PID
gram+ - skin and mucous membrane → pneumonia, sinusitis, cellulitis, osteomyelitis, wound infection, line infection
gram- - GI tract → peritonitis, biliary infection, pancreatitis, UTI, PID
adverse effects of antibiotics
hypersensitivity (allergy) e.g. penicillin
altered normal flora - bacterial overgrowth
drug interactions
specific organ toxicity
antibiotic resistance
allergy types
anaphylaxis - rare but life threatening systemic reaction, develops rapidly and is antibody (IgE) mediated
urticaria - itchy, raised skin and angioedema (local, rapid swelling). also IgE-mediated, more common reactions but less severe
maculopapular rashes - most common allergic reaction but are not life threatening
GI upset cause by penicillin is NOT an allergy
altered normal flora
often occurs when using broad spectrum antibiotics]opportunistic infections may arise
superinfection
candida albicans - oral/vaginal thrush
clostridium difficile diarrhea
drug interactions
tetracyclines or rifampicin and oral contraceptives - reduces effectiveness
ciprofloxacin and aminophylline: this can cause nausea, vomiting, diarrhea, headache, insomnia, seizures or heart palpitations
tissue/organ toxicities
rifampicin and isoniazid long term → liver hepatitis
aminoglycosides e.g. gentamicin → nephro- (kidney) and/or ototoxicity
chloramphenicol → bone marrow depression
antibiotic resistance
commonly used antibiotics are becoming less effective
resistant pathogens threaten our ability to treat common infections and to perform life saving procedures e.g. chemo
antimicrobial stewardship is key in reducing antibiotic misuse to preserve these precious resources for the future