GH - Lecture 5 + GLS
1/93
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
94 Terms
Dominant alleles affect structural proteins, whereas recessive alleles affect enzymes
What makes genes recessive or dominant? (Broad generalisation)
Alteration or truncation of the protein
What may a mutation in an exon cause?
This may change quantity or the pattern of expression
What may a mutation in an intron/promotre sequence cause?
- deletions
- insertions
- base subsitutions
- dynamic transmutations
What are 4 Basic gene mutation classes?
One base pair is replaced with another during replication or repair of DNA
What happens in a base substitution
a deletion from 1 to thousands of base pairs (even multiple genes) - frameshift mutation
What happens in a deletion?
an insertion from 1 to thousands of base pairs (even multiple genes) - frameshift mutation
What happens in an insertion?
Tandem repeats change size following replication
What happens in a dynamic transmutation?
This is a type of mutation where there is a change in base pair but no change in amino acid. (Commonly called a third base wobble)
What is a silent mutation?
In a missense mutation a base pair has been replaced with another, resulting in a different amino acid being produced. Subtypes are: conservative, non-conservative
What is a missense mutation and what are the subtypes?
conservative mutations produce amino acid changes that generally retain some functionality in the protein, whereas non-conservative mutations produce amino acid changes that result in a non functional protein
What is the difference between conservative and non-conservative missense mutations?
This is because in conservative missense mutations tend to swap amino acids with similar properties such as polarity, whereas non-conservative mutations tend to swap amino acids with different properties such as a non-polar amino acid for a polar acidic
Why do some missense mutations conserve function and others don't?
This is a mutation that results in an additional stop codon (UGA, UAG, UAA). This causes a sudden halt in protein translation and usually results in a non-functional chunk of a protein (truncated protein)
What is a nonsense mutation?
This is a mechanism that ensures degradation of nonsense mutation containing mRNA. If an additional stop codon is found, a surveillance complex is formed. This complex causes degradation of the mRNA upon collision with the exon junction complex (EJC).
What is Nonsense mediated decay (NMD)?
g.
abbreviation for genomic level
m.
abbreviation for mitochondrial level
c.
abbreviation for coding DNA
n.
abbreviation for non-coding DNA
r.
abbreviation for RNA level
p.
abbreviation for protein level
A base pair substitution mutation at the DNA/genome level at position 76. An adenine has been replaced with a thymine
interpret g.76A>T
A base pair substitution mutation in RNA at position 76. A guanine has been replaced with a cytosine
interpret r.76g>c
A missense mutation in the protein. At position 26, a threonine has been replaced with a proline
interpret p.Thr26Pro
A missense mutation in the protein at position 77. A Cysteine has been replaced with a Tyrosine
interpret p.Cys77Tyr
A nonsense mutation in a protein. A lysine at position 385 has been replaced with a stop codon
interpret p.Lys385Ter or p.Lys385*
An insertion mutation at the genomic level. A cytosine has inserted between after base 5384 and has taken postion 5385, causing a frame shift for bases 5385 onward.
interpret g.5384_5385insC
A deletion mutation at the genomic level. Base 6174 has been deleted. This causes a frame shift the left for base 6175 oward.
interpret g.6174del
g.13T>C
write the abbreviation at the genome level

p.Leu5Leu
p.Leu5=
write the abbreviation at the protein level

g.12_13insG
Write the abbreviation at the genomic level (base 13 is originially a T)

p.Leu5ValfsTer9
Write the abbreviation at the protein level (AA 5 is usually a Leucine)

- No effect until only very little residual functionality is present
- Disease is present even with minor reduction in functionality
- No effect is seen until a certain level of loss of function which can then be graded in severity
- At a certain level of loss of function one system is affected and at higher levels multiple organ systems become affected
Explain briefly the 4 possible relationships between loss-of-function mutations and clinical phenotype
This is a metabolic disorder in which phenylalanine hydroxylase activity (not presence) is lost. This results in the loss of metabolism of phenylalanine which then is converted into phenylketones. These bodies build up in the body and have neurodegenerative effects
What is Phenylketonuria (PKU)?
PAH gene
Which gene is mutated in PKU?
A non-conservative missense mutation
What kind of mutation causes PKU?
Early symptoms in infants 50%:
- vomiting,
- irritability,
- an eczema-like rash,
- a mousy odour to the urine
Some have:
- increased muscle tone
- more active muscle tendon reflexes
Others:
- microcephaly (small head),
- prominent cheek and upper jaw bones with widely
spaced teeth,
- poor development of tooth enamel,
- decreased body growth
Later:
-cognitive impairment
- seizures
Name some symptoms for Phenylketonuria
Via a phenylalanine level test on a Guthrie card (heel prick)
How are neonates tested for PKU?
Low protein diet, sustaining phenylalanine at lower levels, though some is necessary
What is the best management for PKU?
meat, fish, poultry, eggs, cheese, milk, dried beans, and peas
What are some foods you would tell a pateint with PKU to avoid?
measured amounts of cereals, starches, fruits, and vegetables, along with a milk substitute
What are some foods you would tell a pateint with PKU to eat more of?
When the mother's genetic defects affect the fetus regardless of inheritance
What is the 'maternal effect' in genetics?
A compound heterozygote has 2 defective alleles, though the mutations and defects are different.
What is a compound heterozygote?
Group of heterogeneous blood disorders where one of the globin chains of hemoglobin has not been synthesized correctly.
What is thalassemia?
In alpha thalassemia, the alpha chain is affected, wheras in beta thalassemia the beta chain is affected. There are also epidemiological differences where people from different areas are affected more with one type than the other globally
What is the broad difference between alpha and beta thalassemia?
4 and 2 (beta is coded for by a single gene, alpha is coded for by 2 genes)
How many alleles does a person have for the alpha and beta chain of hemoglobin respectively?
HBA1 and HBA2
What genes code for the alpha chain?
HBB
What genes code for the beta chain?
Throughout foetal development, different kinds of haemoglobin are produced to match changing oxygen demands. Different genes create different hemoglobin chains including Zeta, Epsilon, and Gamma globin. At birth, the individual is still switching from these fetal globins to mature adult globins
Explain why a neonate may be asymptomatic for thalassaemia
-Haemoglobin H (HbH) disease
-Haemoglobin Barts hydrops fetalis (Hb Barts) syndrome
What are the types of alpha thalassemia?
mild form of alpha thalassaemia that causes mild to moderate anaemia that may require treatment
with blood transfusions (either intermittently or on a regular basis), an enlarged spleen and jaundice
(yellowing) of the eyes and skin. 3/4 faulty α globin genes
What happens in HbH disease?
severe form of alpha thalassaemia where excess fluid builds up in the developing baby due to severe anaemia and the baby usually does not survive long after birth. 4/4 faulty α globin genes.
What happens in Hb Barts disease?
-Thalassemia minor (only one gene is defect producing mild life-long anemia)
-Beta Thalassaemia Intermedia
-Beta Thalassaemia Major
What are the types of beta thalassemia?
milder version of beta thalassaemia major, causing mild to moderate anaemia. Symptoms may appear in early childhood or later in life and blood transfusions may be required. Other symptoms include slow growth and bone changes. 2/2 faulty β globin genes
What happens in beta thalassemia intermedia?
severe form of beta thalassaemia. Children develop life-threatening anaemia within the first year of life
and require regular blood transfusions throughout their life. Other symptoms may include failure to thrive, jaundice (yellowing) of the eyes and skin, enlarged spleen, bone changes and developmental delay. 2/2 faulty β globin genes
What happens in beta thalassemia major?
A blood test in which hemoglobin is assessed for by quality and quantity
How is thalassemia screened for?
-Molecular genetic testing of HBA1 and HBA2 detects deletions in about 90% and point mutations in about 10% of affected individuals
-Molecular genetic testing of HBB routinely identifies all variant types including mild and silent mutations
How is thalassemia confirmed/diagnosed?
No effective treatment
What are the treatments for Hb Bart hydrops fetalis?
occasional red blood cell transfusions may be needed during hemolytic or aplastic crises
What are the treatments for HbH disease?
Regular transfusions correct the anemia, suppress
erythropoiesis, and inhibit increased gastrointestinal absorption of iron. The only available definitive cure is bone marrow transplantation from a matched family or unrelated donor or cord blood transplantation from a
related donor.
What are the treatments for Thalassemia major?
Symptomatic therapy based on splenectomy in most affected individuals, sporadic red cell transfusions in some, folic acid supplementation and iron chelation.
What are the treatments for Thalassemia intermedia?
Anemia after 3-18 months. Most die if untreated
When and how will thalassemia major babies usally become affected?
beta, but alpha is rising due to migration from Asian countries
What form of Thalassemia is most common in Australia?
Engaging expression of fetal forms of globin proteins to replace defective adult globins. Potentially gene therapy. Other approaches include: dietary, drugs, and stem cells.
What is a potential future cure for thalassemia thought to be?
A mutation that causes red blood cells to become sickle shaped, ineffective and clump together
What is sickle cell disease in general terms?
HBB gene - The allele in sickle cell disease is called HBS
Which gene is affected in sickle cell anemia?
p.Glu6Val
What is a common mutation in sickle cell disease?
Generally done by viewing blood sample microscopically
How is sickle cell disease diagnosed?
SIckle cell disease is prevalent in populations with high levels of malaria, as the misshapen RBCs stop the spread of malaria and provide a protective advantage, particularly in heterozygotes where normal function is maintained
Provide a theory for why sickle cell disease is more prevalent in Africa
G6PD deficiency (parasite cannot reproduce)
What is another genetic disease that provides a heterozygote advantage against malaria?
PKU (phenylketonuria) (elevated phenylalanine inactivates fungal toxin)
What genetic mutation is protective against foetal fungal infection in heterozygotes?
Prion Protein Mutation (prion protein cannot misfold in presence of infections prion protein)
What genetic mutation is protective against transmissible spongiform encephalopathy in heterozygotes?
cystic fibrosis (fewer functional CFTR channels for cholera to affect)
What genetic mutation is protective against cholera in heterozygotes?
Smith-Lemli-Opitz Syndrome (SLOS) (Lowered serum cholesterol)
What genetic mutation is protective against cardiovascular disease in heterozygotes?
Good outlook if routinely checked and crisis prevented
What is the prognosis for sickle cell disease?
The body absorbs and stores too much iron which damages organs
What happens in haemochromatosisin general terms?
HFE gene (High Fe - iron)
Which gene is affected in haemochromatosis?
p.Cys282Tyr
What is the most common mutation that causes haemochromatosis?
Europeans - From medieval Celtic origin
Which demographic is largely affected by haemochromatosis?
Hepcidin - master iron regulatory hormone (regulation is affected)
What is the hormone that is affected in haemochromatosis?
Joint pain, fatigue, lack of energy, abdominal pain, loss of sex drive, heart problems, irreversible organ damage, bronze diabetes (darkened skin due to iron, hyperglycemia)
What are some of the signs and symptoms for haemochromatosis?
phlebotomy (bleeding)
What is one way to treat haemochromatosis?
A mutation causes the cystic fibrosis trans-membrane conductance regulator protein to not move chloride as efficiently, causing a lack of fluid and thickened mucus in the lungs and digestive tract. Other organs also affected
What happens in cystic fibrobsis in general?
A gland which secretes digestive enzymes for fat into the GI tract becomes blocked and cysts form eventually becoming fibrous (scarring). This also means that there aren't enough fat digesting enzymes in the gut, causing malabsorption and fatty stool
What happens to the pancreas in CF?
Salts from sweat are not taken back up and a salty lining is left on the skin and deficit in the body
What happens to sweat metabolism in CF?
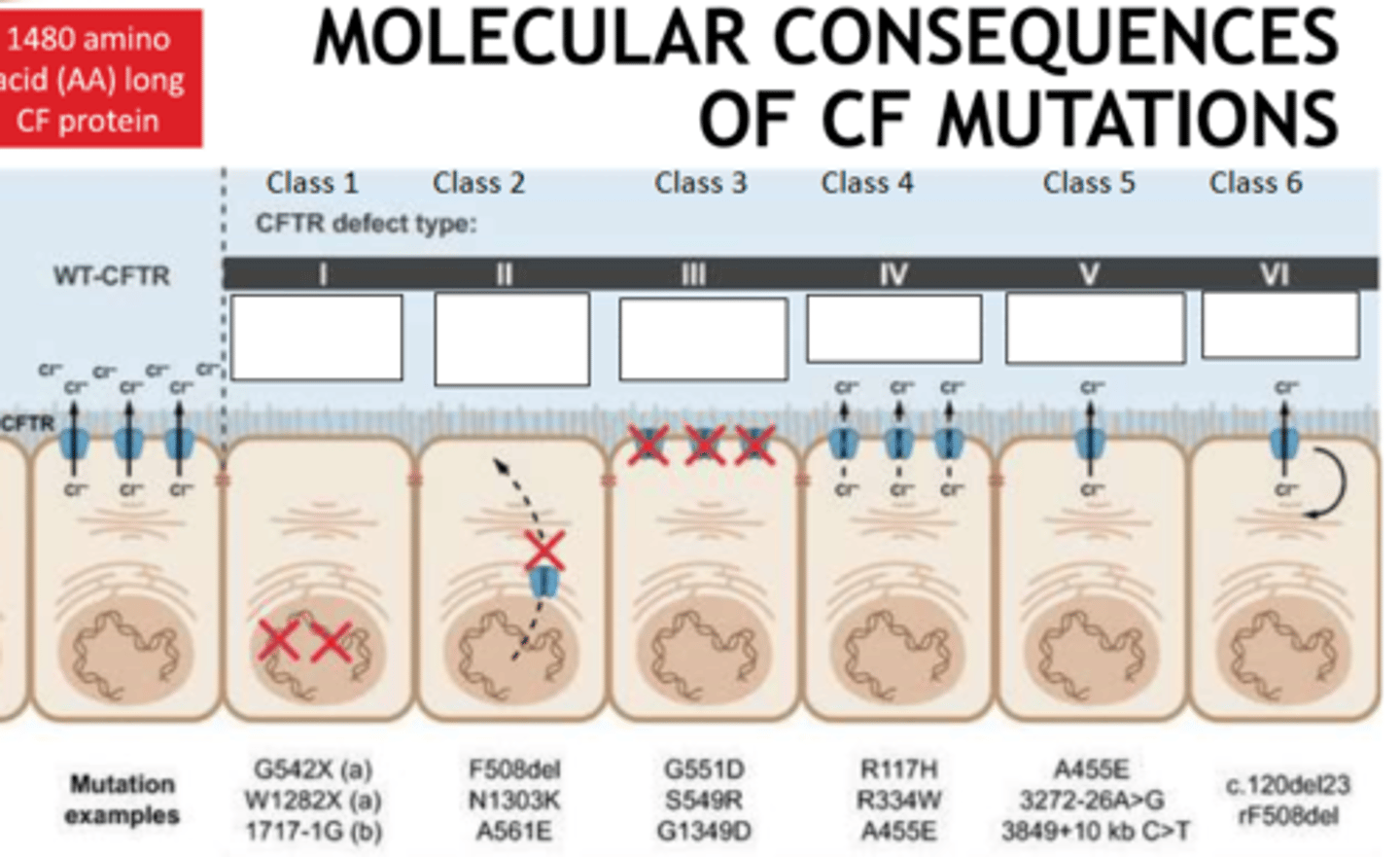
Delta F508 mutation (p.Phe508del)
What is the most common CF mutation?
>2000
How many other mutations are there for CF?
p.Trp128*
p.Gly542*
p.Gly551Asp
p.Asn1303Lys
What other mutations are common in CF?
6
How many classes of CF mutation are there?
- No protein
- no traffic (not embedded in cell membrane)
- no function
- less function
- less protein
- less stable (targeted by ubiquitine proteosome pathway excessively)
Fill the blanks
Screening: population level, assess risk only - Guthrie card
Diagnostic: individual level, confirmatory - genome sequence
What is the general difference between screening tests and diagnostic tests for genetic disease?
IRT (immunoreactive trypsinogen) from pancreas
What is the marker screened for in CF?
Medication: antibiotics, mucus thinners, bronchodilators, pancreatic enzymes
Physical therapy: pounding chest and back to mobilise mucus in lungs
Organ transplant: lungs
Name some treatments for CF
Physio every day for a few hours,
many pills every day (up to 40),
if possible physical exercise helps,
big focus on nutrition due to malabsorption,
50% of patients live past 41 years old
What is the outlook and quality of life like for people with CF?
Respiratory failure due to chronic bacterial infection
What is the most common cause of death in people with CF?