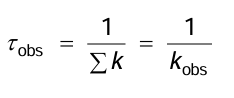Electronic States 1
1/152
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
153 Terms
What is photochemistry?
The chemistry of reactions that are initiated by light.
What happens when a photon is absorbed?
It creates and excited electronic state, allowing chemistry to occur
What happens when an electronic transition occurs?
An electron moves from one MO to another, causing redistribution of electron density, therefore changing the bonding and structure.
How are electronic transitions induced?
Typically by UV-visible light at wavelengths around 200-800nm
What is a chromophore?
Absorbers of UV-visible light
Why is the sun important for spectroscopy?
It is a natural source of photons which are required for photochemistry and colouration.
What effect do unsaturated systems have on the absorption of photons?
When there is increasing conjugation, the absorption shifts to longer wavelengths
What is the ground state?
The lowest energy electronic state in which electrons occupy bonding and non bonding MOs
What happens when a photon is absorbed?
It induces an electronic transition in which an electron is promoted to a higher energy MO (usually antibonding), creating an excited state.
How can two different excited states occur but occupy the same MOs?
If the electrons occupy the orbitals with different spin.
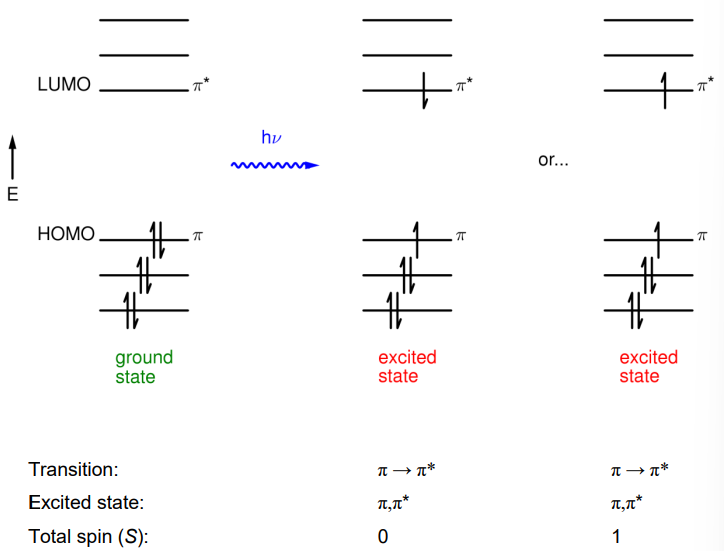
How are the electron spin properties of a state defined?
Described by their multiplicity, which is defined as 2S+1, where S is the total spin.
How is the excited state written as?
Where the transition is written inside the brackets.
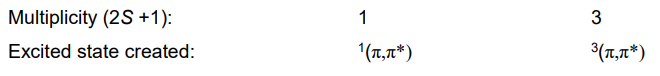
What are state diagrams?
Used instead of orbital diagrams, showing the energy states of the whole molecule (individual electrons not shown)
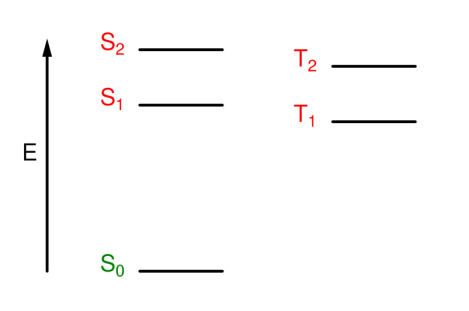
What are the state labels?
Singlet = S
Triplet = T
Ground = 0
Excited = 1, 2, 3...
What is the S state?
The singlet state, where 2S+1=1.
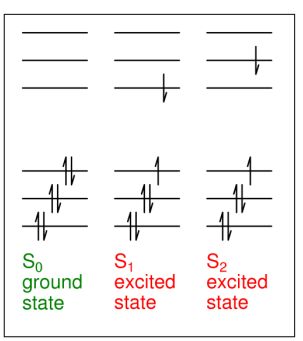
What is the T state?
The triplet state, where 2S+1=3
How are the singlet state and triplet state related?
Each excited singlet state has an equivalent excited triplet state at a lower energy (Hund's rule)
Why is there no T0 state?
Due to Pauli's exclusion principle, you cannot have two electrons with the same spin in the same orbital.
What UV-Visible transitions are possible for conjugated organic molecules?
Pi -> Pi*
n.b -> Pi* (if lone pairs are present)
What other transition can conjugated organic molecules have?
n.b -> Sigma*
Pi -> Sigma*
Sigma -> Pi*
Sigma -> Sigma*
These all give bands at high energies, at wavelengths under 200nm.
What do the UV-Vis spectra of atoms look like & why?
Consists of sharp lines due to the clearly defined electronic energy levels. Atoms have no rotational or vibrational degrees of freedom.
What do the UV-Vis spectra of molecules look like?
Consists of many lines, or broad bands due to each electronic state having many energy levels. Molecules do have rotational and vibrational degrees of freedom.
What is the Born-Oppenheimer approximation?
The electronic, vibrational and rotational properties are considered to be independent of each other.
What is the total wavefunction equation?
Multiply the electronic, vibrational & rotational wavefunctions.
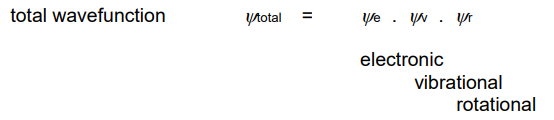
What is the total energy equation?
Sum of the electronic, vibrational & rotational energies.
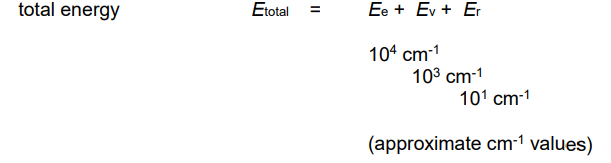
What does the longest wavelength absorption arise from?
The lowest energy transition, from S0 to the S1 excited state.
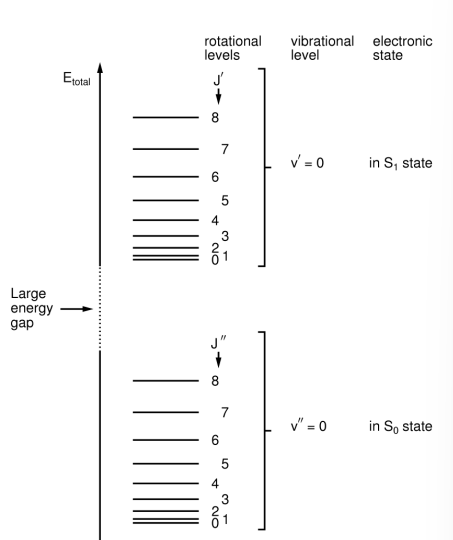
Where do electronic energy transitions occur from?
From the ground state (S0) to the S1 excited state.
Where do vibrational energy transitions occur from?
From the electronic ground state (S0) in v'' (the lowest vibrational state), to several v' (upper vibrational state) levels in the S1 state.
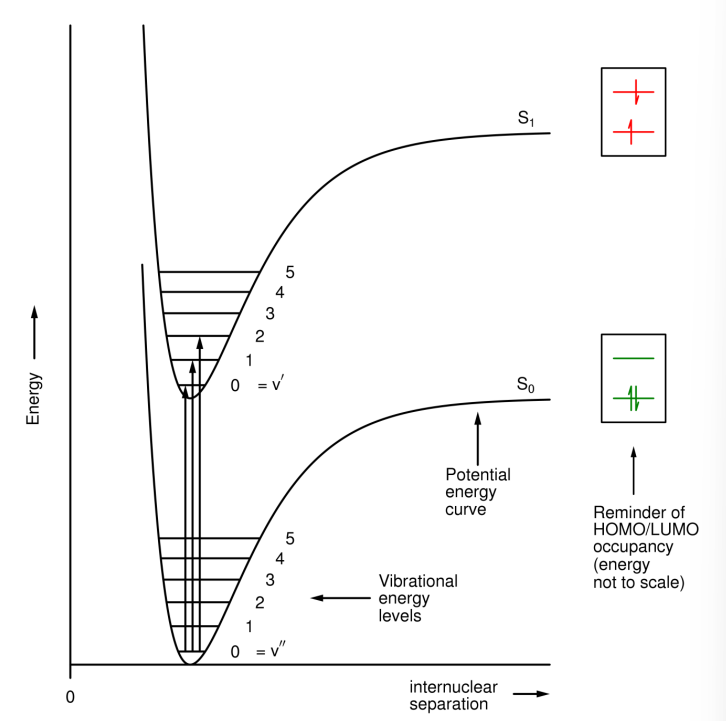
Where do rotational energy transitions occur from?
From several J'' (lower rotational energy state) levels in the v''=0 level to several J' levels in each v' level in the S1 state.
How is the rotational fine structure seen?
From gases at high spectroscopic resolution.
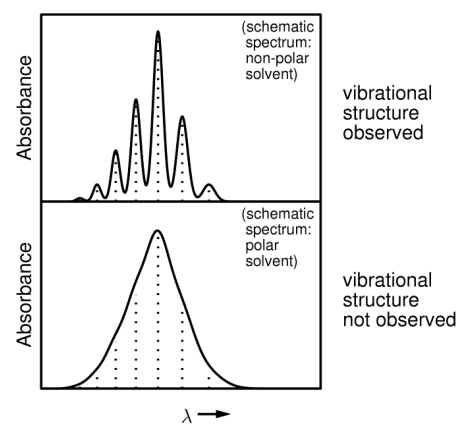
How is the vibrational fine structure seen?
From gases, also liquids and solids in some cases. However the peaks can be broad, particularly in polar solvents (due to strong interactions)
What determines the required energy of the photon?
Must be at least the same energy as the energy gap between ground and excited states.
What is the strength of the absorption coefficient determined by?
The selection rules.
What is the overall selection rule for an electronic transition?
To excite an electron from one orbital to another, the electric field of the light must cause a displacement charge, giving a transition dipole moment (TM)

What is the equation given after applying the Born-Oppenheimer approximation and what does it tell us?
It gives three separate selection rules (shown by the integrals).
If any of the 3 integrals is 0, then the TM is 0 and the transition is disallowed by that selection rule.
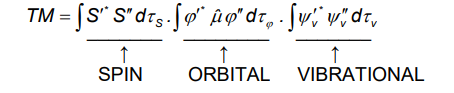
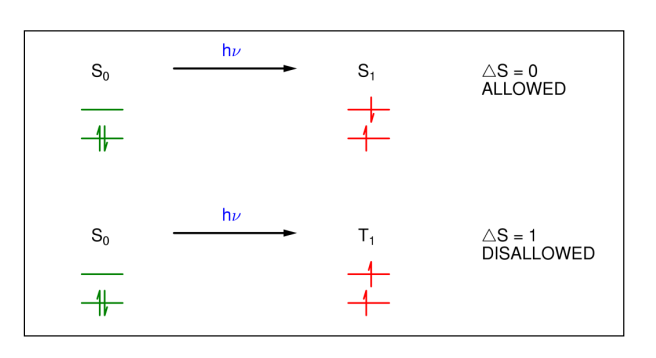
What is the spin selection rule?
An electron has two possible spin states, up (a) and down (B), for which the wavefunctions are either normalised or orthogonal.
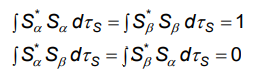
What does the spin selection rule mean?
For the TM to be non-zero, the electron must retain its spin (change in S=0), so there is no change in multiplicity.
What is the orbital selection rule?
The orbital selection rule has two parts, symmetry and spatial.
What is the symmetry part of the orbital selection rule?
The TM can be separated into components along the x, y & x axes.
To be symmetry forbidden, all 3 components must be 0.
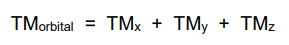
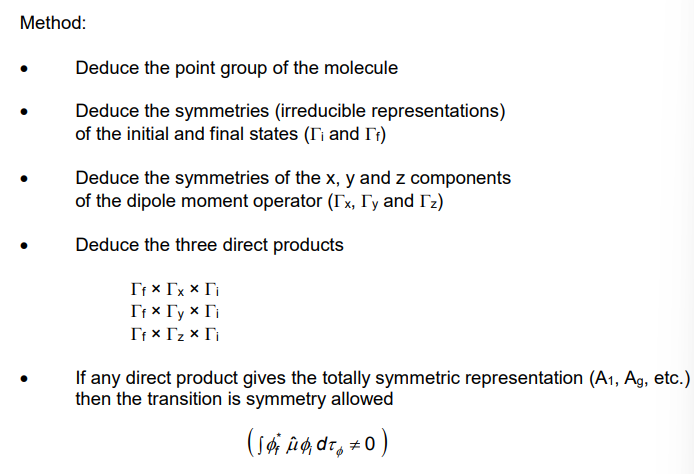
How is group theory used to decide if it is symmetry forbidden?
The products must contain atleast 1 A1.
What is the spatial part of the orbital selection rule?
If the initial and final orbitals must occupy a region of space that is similar as the spatial overlap integral will be large & therefore spatially allowed.
What is the most probable geometry given by?
The highest wavefunction^2.
For v=0, it is at the equilibrium geometry (re).
For v>>>0 it is close the the PE curve (the turning points).
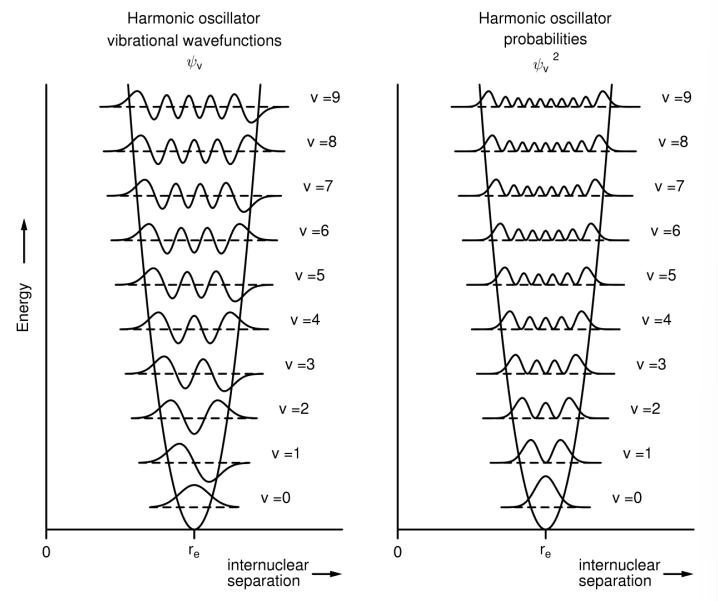
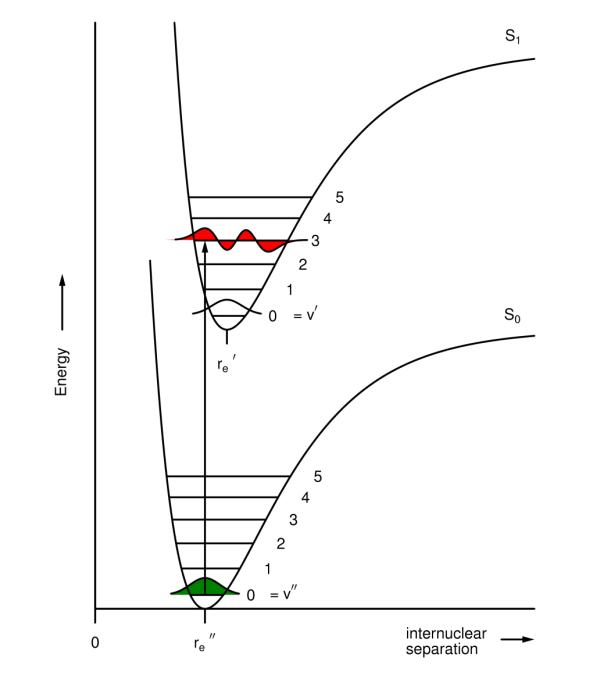
What is the Franck-Condon principle?
Electrons move faster than nuclei due to their relative masses.
Why do electronic transition occur mainly from v''=0 to v' levels that give large vibrational overlap integrals?
Because there is no change in the positions of the atoms during the transition, any change in geometry occurs after the transition. This is explained by the Franck-Condon principle.
Why is re(S1) often larger than re(S0)?
If an electron is being promoted to an antibonding orbital, the bond length increases as the bond is weaker.
What is the probability of transition given by?
The oscillator strength (f).
The oscillator strength relates experimental ε values to the selection rules.

What does oscillator strength relate to?
It relates the selection rules to experimental ε values.

What is oscillator strength given as?
Where f is oscillator strength.
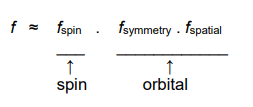
What values of f are allowed?
Where 'allowed' means high ε value.

What is spin-orbit coupling?
Spin & orbital motions of an electron are not independent, so there is some coupling.
The magnitude of SOC is proportional to z^4.
When would a 'spin-flip' transition occur?
It is strongly forbidden, however it is weakly allowed in the presence of heavy atoms (large z).
This can be internal heavy atoms, or external (e.g. in the solvent).
What is vibronic coupling?
Vibrational and electronic motions are not independent, so they can couple.
When would a forbidden electronic transition be allowed?
Can be weakly allowed if the point group changes (if symmetry lowers).
This may happen if there is an asymmetric vibration distorting the molecule.
What is photophysics?
The non-reactive decay of excited states.
Why are excited states short-lived?
They have a large excess of energy.
By what processes do excited states return to the ground state?
Radiative energy loss.
Non-radiative energy loss.
What is vibrational relaxation (vr)?
An excited electronic state is often formed in a vibrationally excited state, so the excess vibrational energy is given to surrounding molecules by collisions.
What type of process is vibrational relaxation?
Non-radiative.
Why does vibrational relaxation usually occur before other processes?
The rate constant in liquids is very high.
What is luminescence?
The general name for a radiative transition.
What is fluorescence?
A radiative transition between states of the same multiplicity.
What are the selection rules for fluorescence?
The same as for electronic absorption:
Spin
Orbital
Vibrational overlap integral
Is fluorescence spin allowed?
Yes, the rate constant is typically high:
kf ≈ 10^6 - 10^9s^-1
What does the fluorescence spectrum show and how?
It shows a vibrational progression from S1 (v'=0) to several S0 (v'') levels.
The probabilities are determined by vibrational overlap integrals.
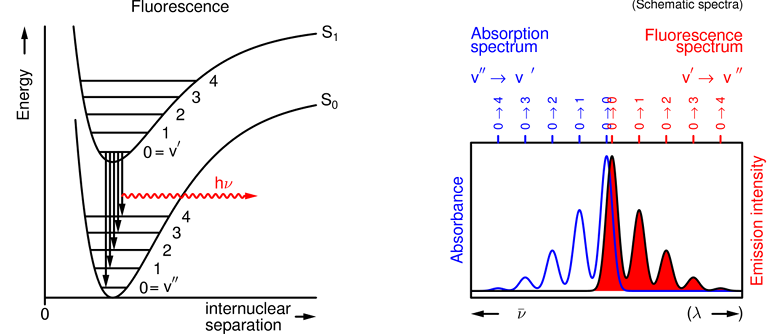
What does the spacing between the individual peaks show in a fluorescence spectrum?
It gives the spacing of the vibrational energy levels in the final state.
Absorption shows the v' levels in the S1 state, whereas emission gives the v'' levels in the S0 state.
Why are the spacings in the fluorescence absorption & emission spectra different?
The vibrational energies are different in the S1 & S0 states- they are typically smaller in the S1 state as the bonds are weaker.
Why is vr slower for gases in the fluorescence spectrum?
The collision rate is much lower than in liquids, making vr slower.
Unrelaxed fluorescence can be observed from higher v' levels in gases.
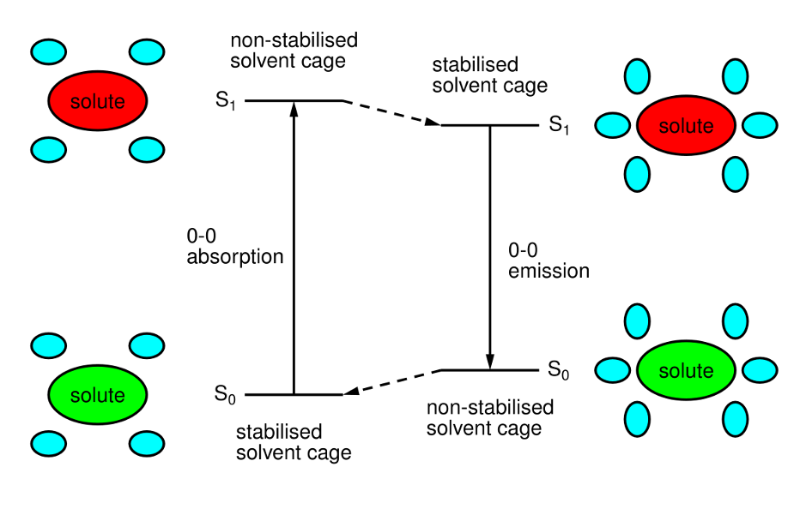
Why is the 0-0 band shifted to lower energy in the fluorescence emission spectra compared to the absoprtion?
Due to 'Stokes shift', the solvent rearranges to stabilise the excited state after absorption, but before emission. The extent of stabilisation varies with molecule and solvent (polar systems typically give large shifts).
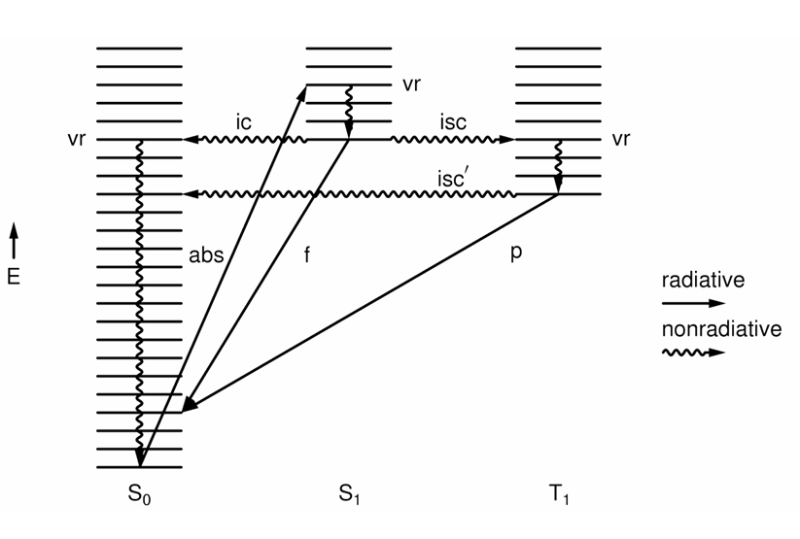
What is internal conversion (ic)?
A non-radiative, isoenergetic transition between states of the same multiplicity.
This means the molecule changes state by an excited electron moving to a lower MO with ΔEtotal = 0 and ΔS = 0 (no change in total energy but the state formed is vibrationally excited).
Vr follows rapidly.
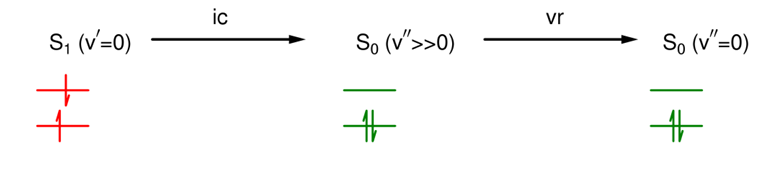
What are the selection rules for non-radiative transitions?
They are similar to those for radiative transitions:
Spin
Orbital
Vibrational overlap integral
Is internal conversion spin allowed?
Yes
What does the vibrational overlap integral depend on?
How the states differ in energy and equilibrium geometry (re).
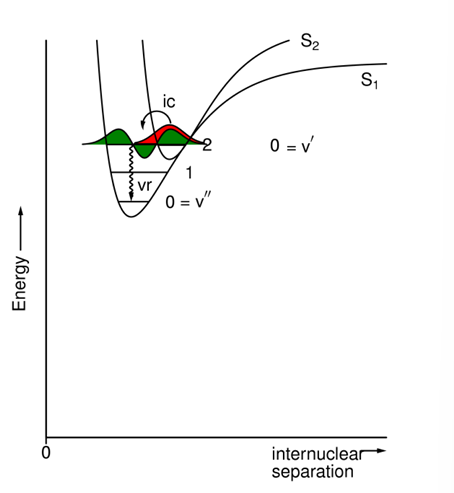
What does the energy diagram for an internal conversion between different excited states look like?
Typically, between different excited states, the energy gap is small, the overlap integral is large & the rate constant is very large (10^12 - 10^13 s-1).
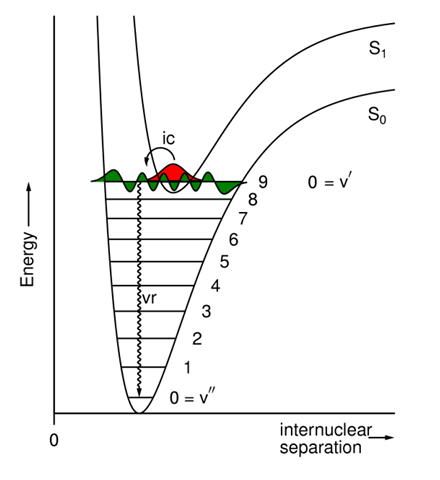
What does the energy diagram for an internal conversion between excited & ground states looks like?
Between excited & ground states, the energy gap is large, the overlap integral is small, & the rate constant is large (10^5 - 10^9s-1).
What is Kasha's rule?
If higher excited states are created, the ic & vr occur very rapidly.
Fluorescence and other processes usually occur exclusively from the S1 state.
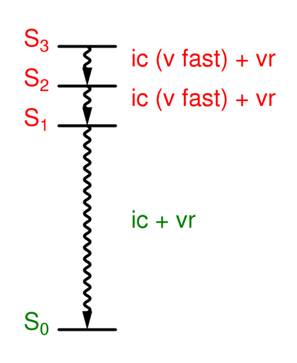
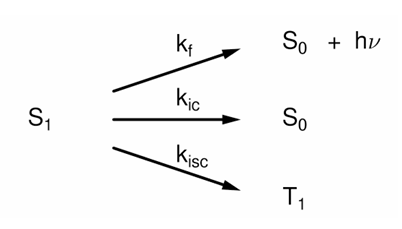
What happens when ic and f are in competition in the S1 state?
The relative yields depend on the relative rate constants.
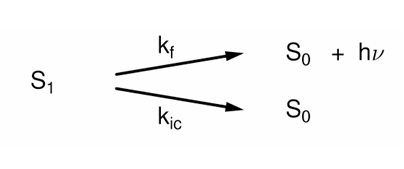
What is intersystem crossing?
A non-radiative isoenergetic transition between states of different multiplicity.
This means the molecule changes state by an electron spin flip, and may also change MO simultaneously. There is no change in total energy.
Vibrational relaxation follows rapidly.
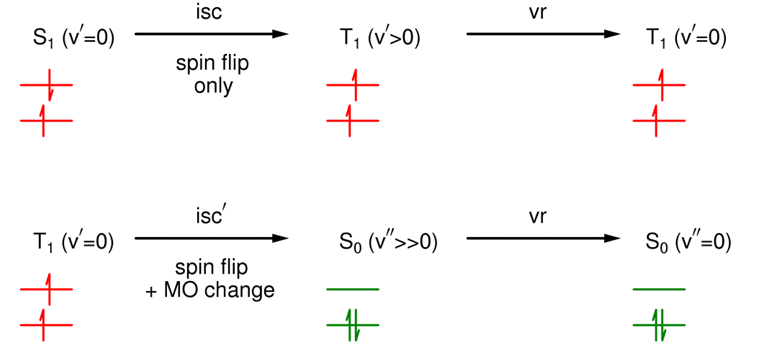
Is intersystem crossing spin allowed?
No (spin flip).
What does the vibrational overlap integral depend on for internal conversion?
It depends on how the states differ in energy and equilibrium geometry (re).
How do the relative rate constants of intersystem crossing and internal conversion compare?
kisc << kic
Due to the spin selection rule.
What does the energy diagram for intersystem crossing look like between different excited states?
Typically, the energy gap is small, the overlap integral is large, & the rate constant is large (10^6 - 10^11s-1).
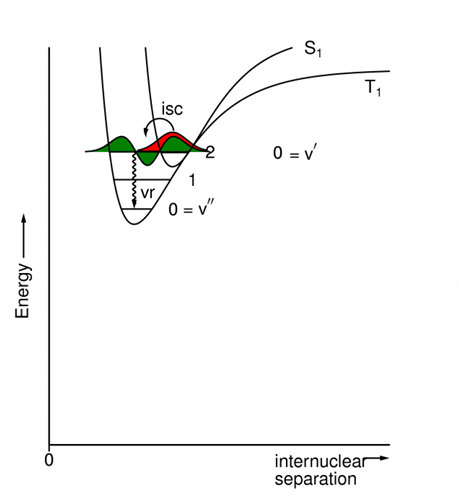
What does the energy diagram intersystem crossing look like between excited and ground states?
Typically, the energy gap is large, the overlap integral is small, & the rate constant is very small (10^-3 - 10^3s-1).
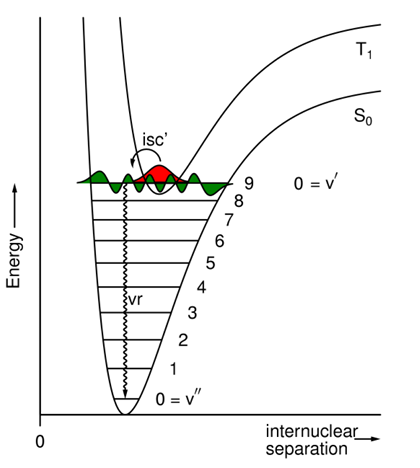
What does isc compete with in the S1 state and what determines the yields?
Isc competes with f & ic.
The relative yields depend on the relative rate constants.
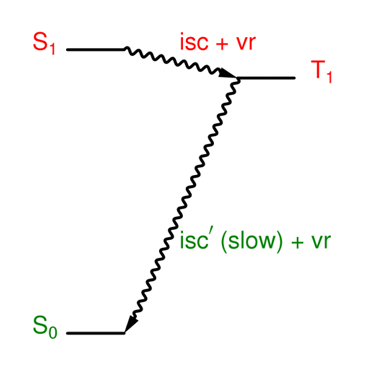
Why is isc important for many molecules?
It is an efficient way to create T1 states, which are relatively long lived.
What is phosphorescence (p)?
A radiative transition between states of different multiplicity.
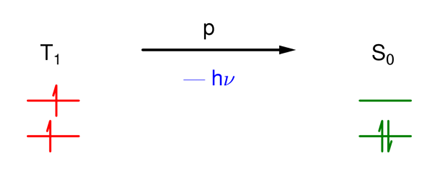
Is phosphorescence spin allowed?
No.
The rate constant is therefore typically low (10^-3 - 10^4s-1)
What does the phosphorescence spectrum show?
A vibrational progression from T1 (v'=0) to several S0 (v'') levels.
It's profile is usually similar to the fluorescence spectrum as vibrational overlap integrals are usually similar, however phosphorescence is at a longer wavelength (the T1 state is at lower energy than the S1 state).
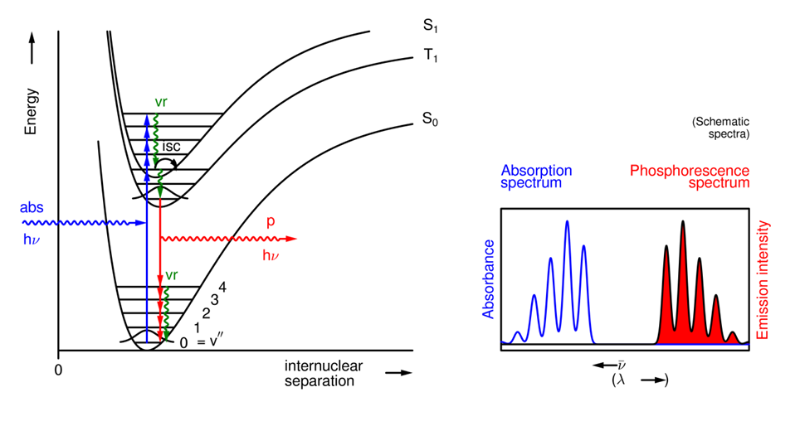
What is a Jablonski diagram?
A simple schematic diagram that summarises photophysical processes.
The energy is the vertical axis (no parameter as the horizontal axis).
What is chemiluminescence?
Luminescence from an excited state formed as a product of a chemical reaction. To release enough energy, it is required to be a strongly exothermic reaction.
What is the quantum yield (Φ) given by?
This is applicable to products of photochemical reactions & photophysical processes.

What is the quantum yield for ic given by?
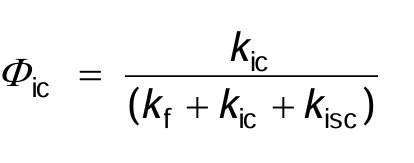
What is the quantum yield for isc given by?
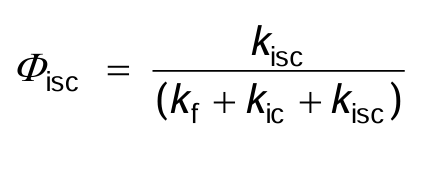
What are quantum yields determined by?
How the rate constant for a specific process competes with the sum of the rate constants for all the decay processes.
What should you consider for a process from the T1 state?
The quantum yield of T1 formation.
The fraction of molecules in the T1 state that decay by the process (the quantum efficiency, θ)
What is quantum efficiency (θ)?

What is the quantum yield for isc'?
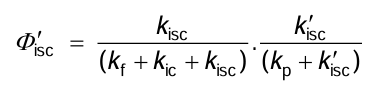
What are the quantum yields from T1 states determined by?
The competition between all photophysical decay processes, not only those from T1.
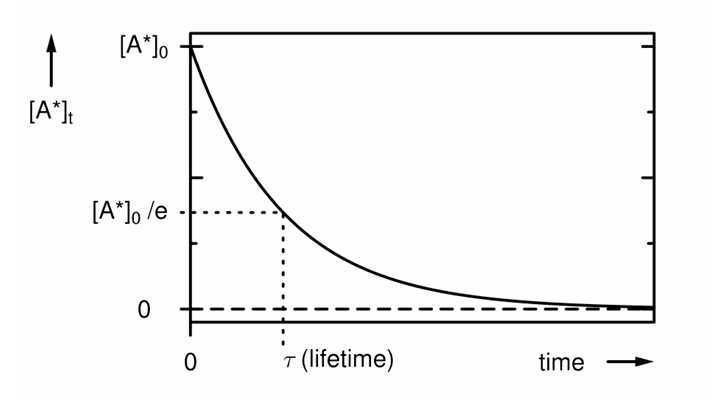
What is the lifetime defined as?
The time when [A*] falls to 1/e of its initial value.
![<p>The time when [A*] falls to 1/e of its initial value.</p>](https://knowt-user-attachments.s3.amazonaws.com/0676d1d2-02f5-4a4c-ac05-27c6e745bbd6.png)
How do you find lifetime on a graph?
What is the observed lifetime?
The lifetime as measured by the experiment.