W4L10: Unimolecular nucleophilic substitution SN1
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
9 Terms
Nucleophilic substitutions
In any case the electrophile must be hybridised sp3
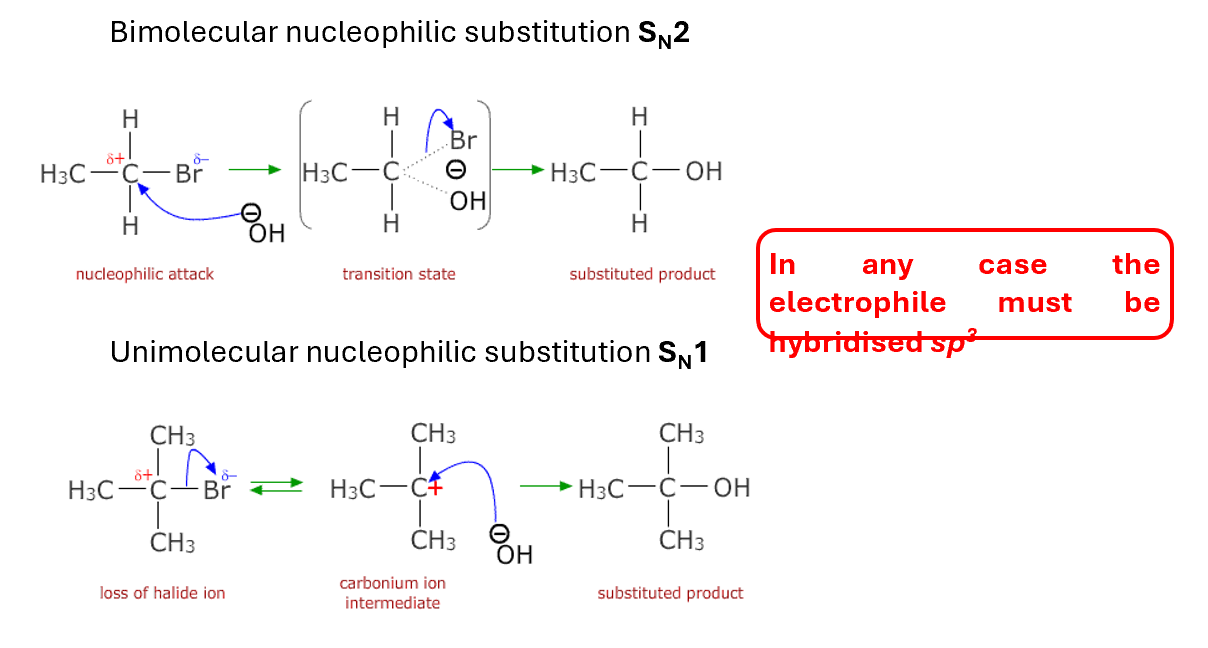
For any nucleophilic substitution we need to keep into consideration:
Structure of the reagent (halogeno-alkanes are very common)
Type of solvent (polar aprotic, polar protic…)
Strength of the nucleophile
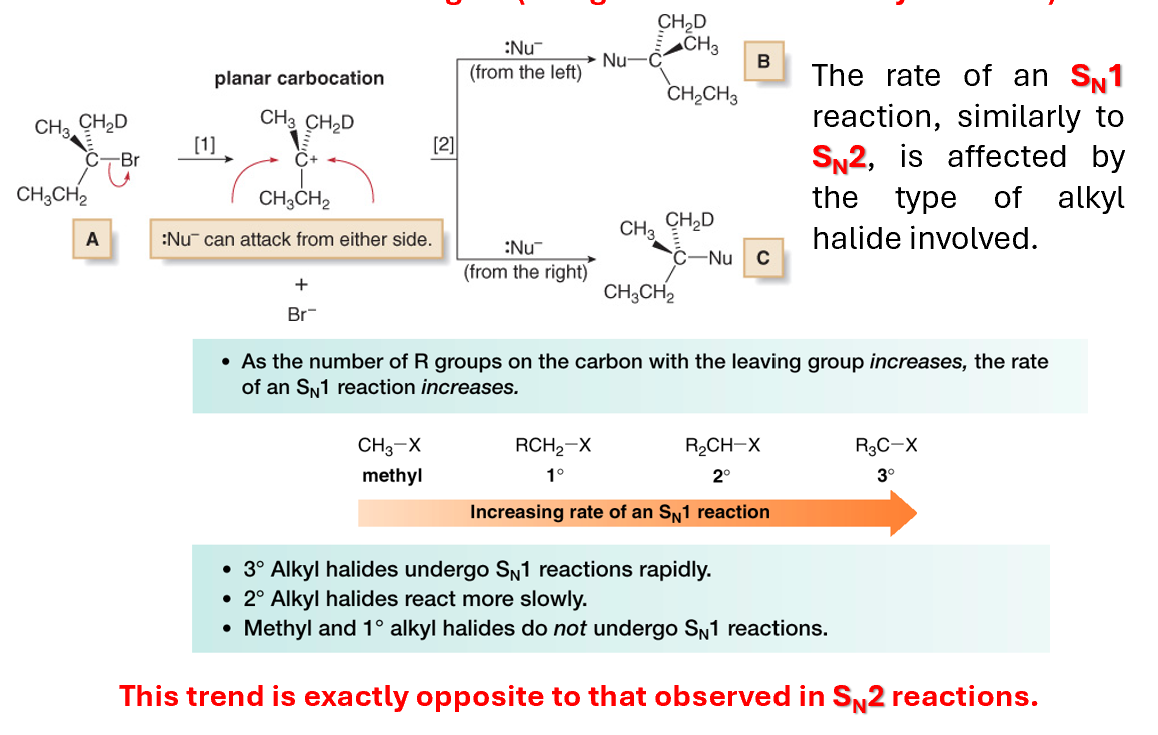
Number of molecules involved in the transition state (Rate of the reaction)
Leaving group (LG)
Stereochemistry of the substitution
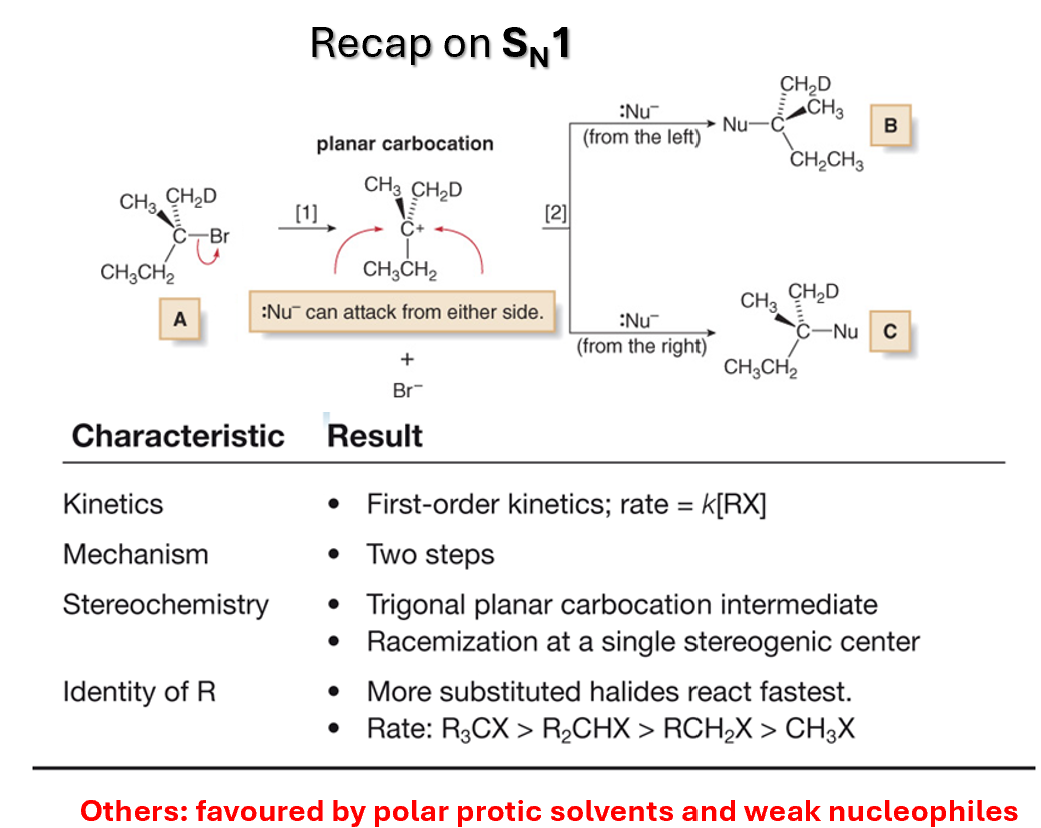
Strength of the nucleophile influencing SN1
Since nucleophilic addition occurs after formation of carbocation, reaction rate is not normally affected by nature or concentration of nucleophile
In general, though, if there is competition between SN1 and SN2, strong nucleophiles tends to favour the SN2 mechanism, weak nucleophiles SN1
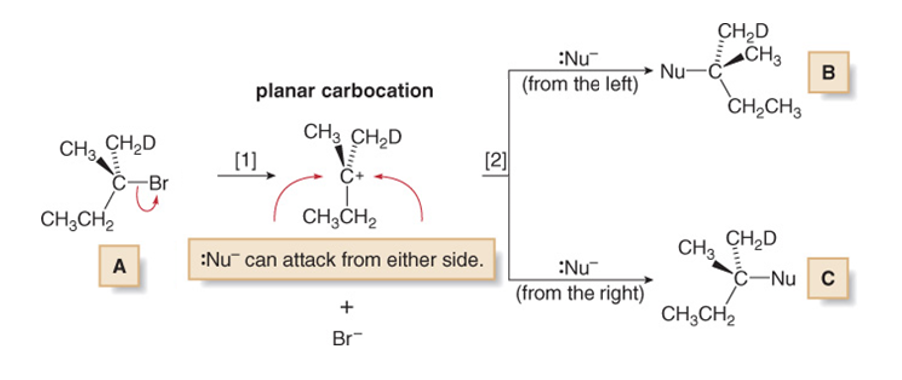
Structure of the reagent influencing SN1
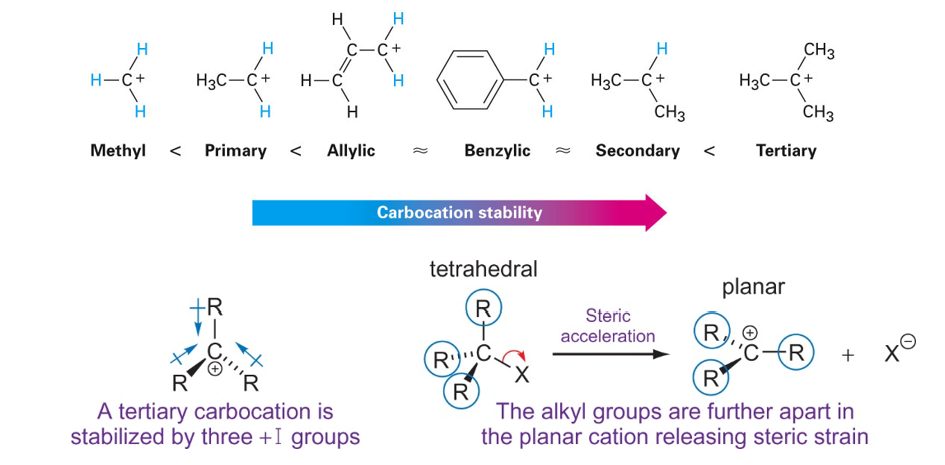
Stability of carbocation influencing SN1
The order of carbocation stability can be rationalized through inductive effects and hyperconjugation
Hyperconjugation is the spreading out of charge by the overlap of an empty p orbital with an adjacent pi bond. This overlap (hyperconjugation) delocalizes the positive charge on the carbocation, spreading it over a larger volume, and this stabilizes the carbocation
Inductive effects are electronic effects that occur through bonds. Specifically, the inductive effect represents the influence of the electron density through bonds caused by electronegativity of the connecting group.
In general, the greater the number of alkyl groups attached to a carbon with a positive charge, the more stable will be the cation
Solvent influencing SN1
The Hammond postulate relates reaction rate to stability. It provides a quantitative estimate of the energy of a transition state.
The Hammond postulate states that the transition state of a reaction resembles the structure of the species (reactant or product) to which it is closer in energy.
Polar Protic solvents favour the SN1 reaction. The formation of hydrogen bonding between the solvent and the carbocation stabilizes the intermediate.
Consequence of the Hammond postulate: Any factor that stabilizes a high-energy intermediate stabilizes the transition state leading to that intermediate
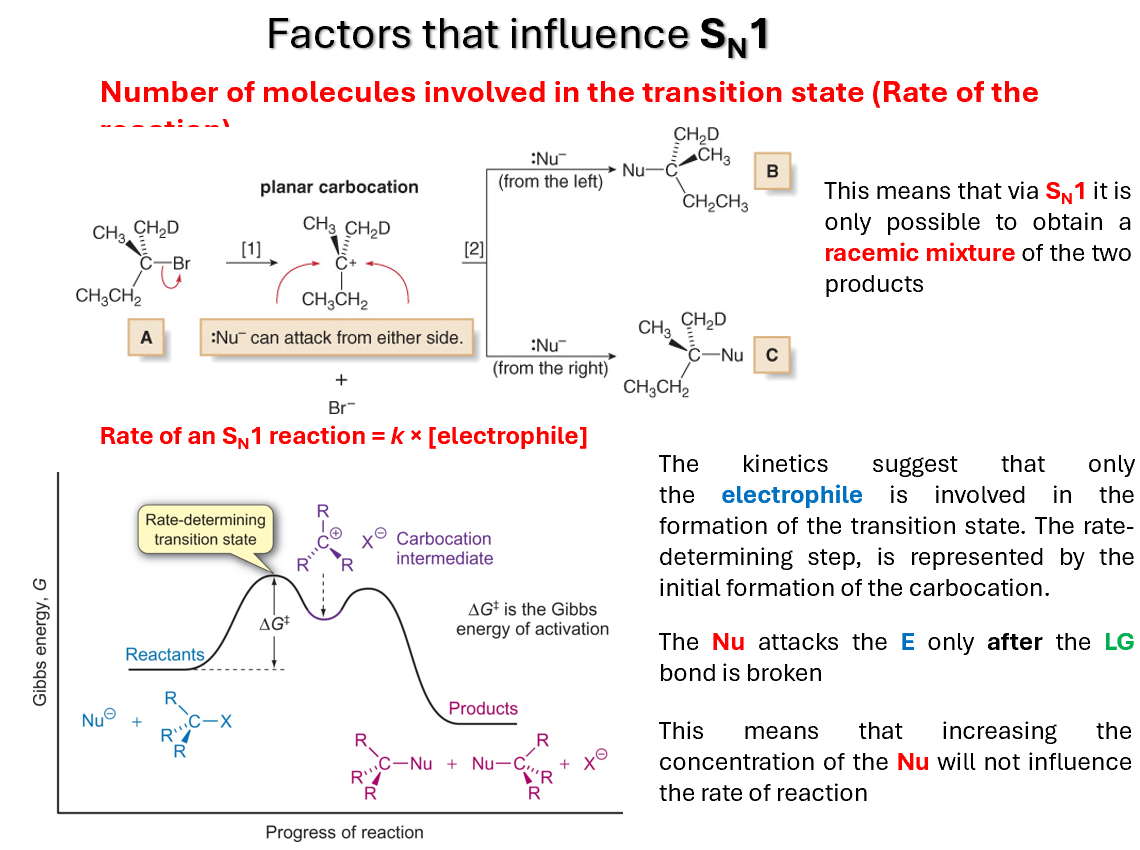
Number of reactants influencing SN1
The kinetics suggest that only the electrophile is involved in the formation of the transition state. The rate-determining step, is represented by the initial formation of the carbocation.
The Nu attacks the E only after the LG bond is broken
This means that increasing the concentration of the Nu will not influence the rate of reaction
This means that via SN1 it is only possible to obtain a racemic mixture of the two products
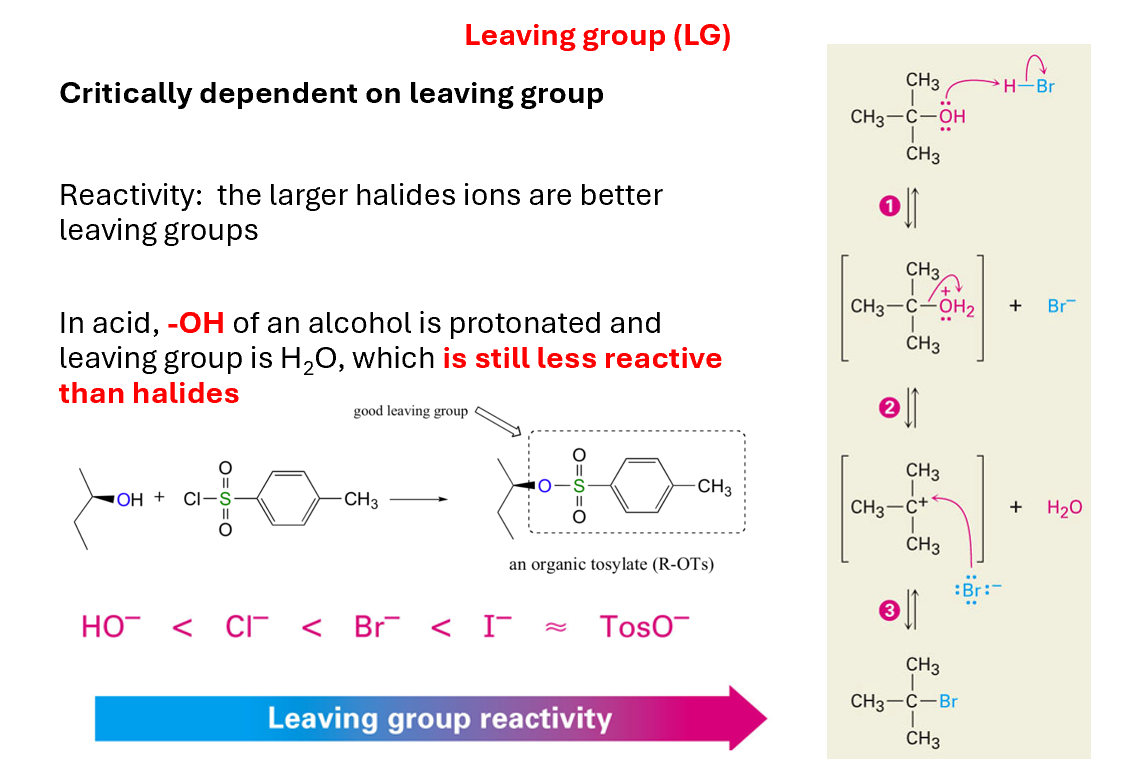
LG influencing SN1
Critically dependent on leaving group
Reactivity: the larger halides ions are better leaving groups
In acid, -OH of an alcohol is protonated and leaving group is H2O, which is still less reactive than halides
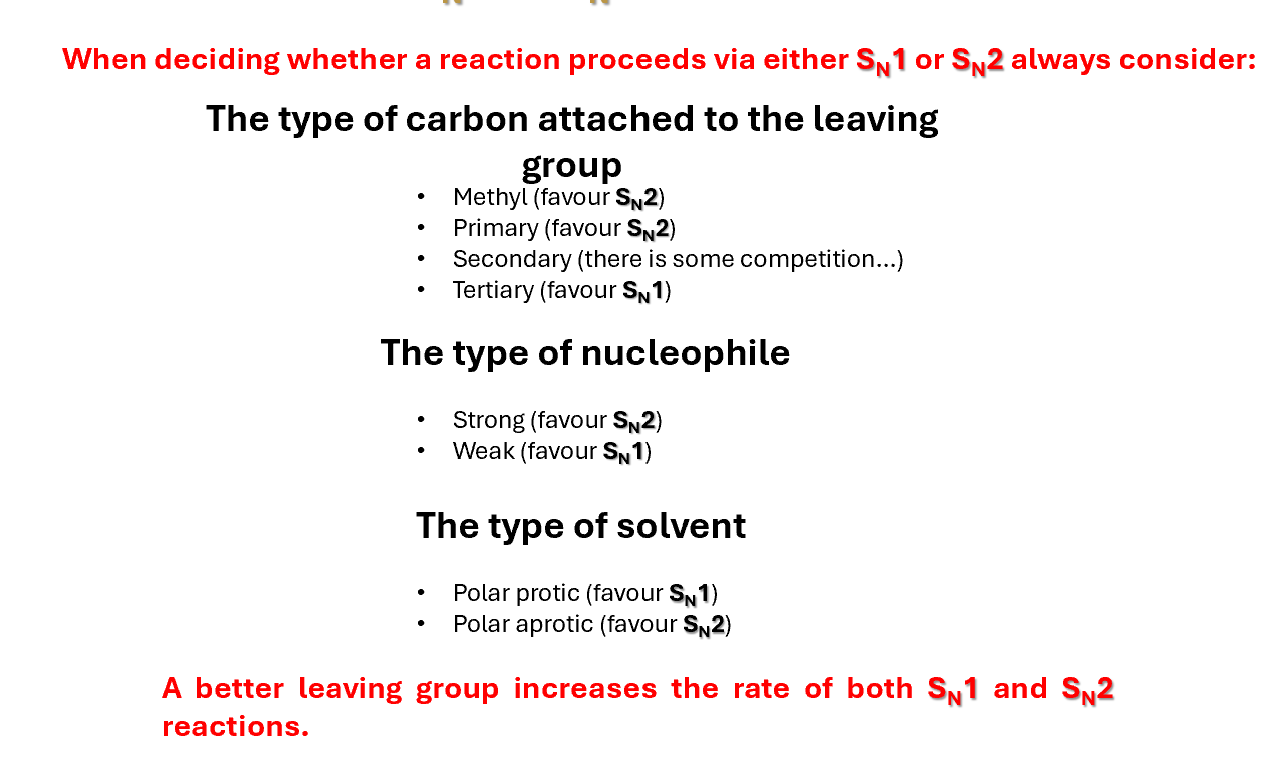
SN1 or SN2