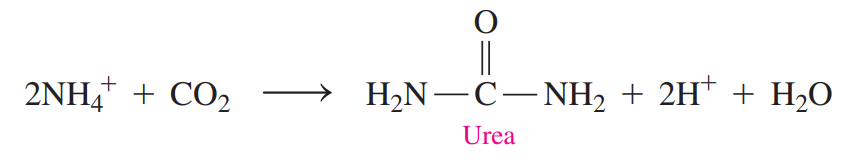Chapter 18: Metabolic Pathways and Energy Production
18.1: Metabolism
Metabolism: All the chemical reactions that provide energy and the substances required for continued cell growth.
Catabolic Reactions: These are complex molecules that are broken down into simpler ones with an accompanying release of energy.
Anabolic Reactions: These utilize the energy available in the cell to build large molecules from simple ones.
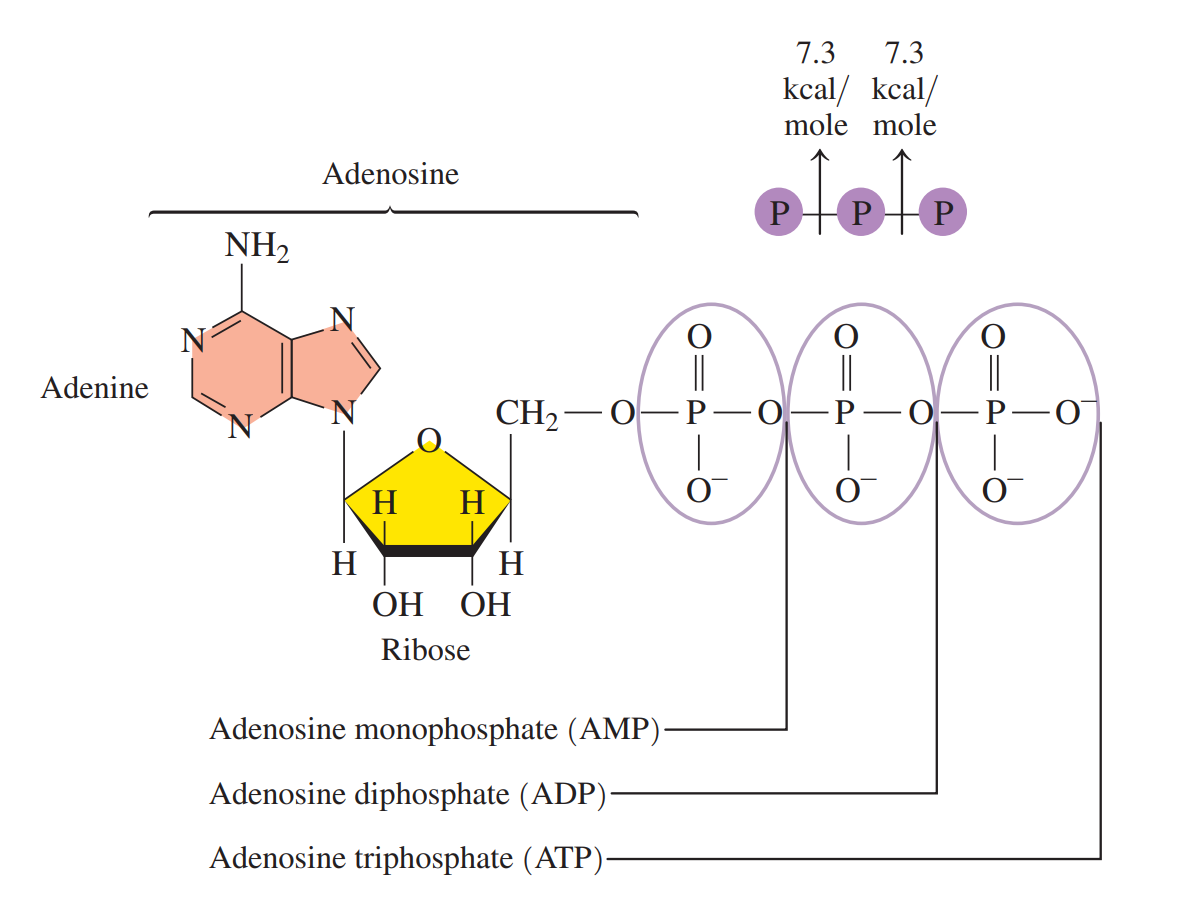
Adenosine Triphosphate (ATP): A high-energy compound that stores energy in the cells. It consists of adenine, a ribose sugar, and three phosphate groups.
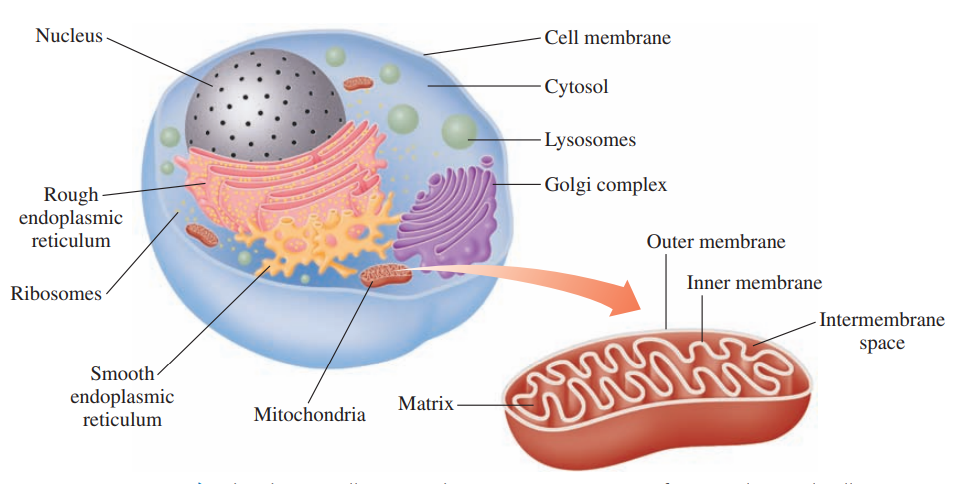
Cell Structure for Metabolism
Cell membrane: It separates the contents of a cell from the external environment and contains structures that communicate with other cells.
Cytoplasm: It consists of the cellular contents between the cell membrane and nucleus.
Cytosol: It is the fluid part of the cytoplasm that contains enzymes for many of the cell’s chemical reactions.
Endoplasmic reticulum: It is the rough type that processes proteins for secretion and synthesizes phospholipids; smooth type synthesizes fats and steroids.
Golgi complex: It modifies and secretes proteins from the endoplasmic reticulum and synthesizes cell membranes.
Lysosome: It contains hydrolytic enzymes that digest and recycle old cell structures.
Mitochondrion: It contains the structures for the synthesis of ATP from energy-producing reactions.
Nucleus: It contains genetic information for the replication of DNA and the synthesis of protein.
Ribosome: It is the site of protein synthesis using mRNA templates.
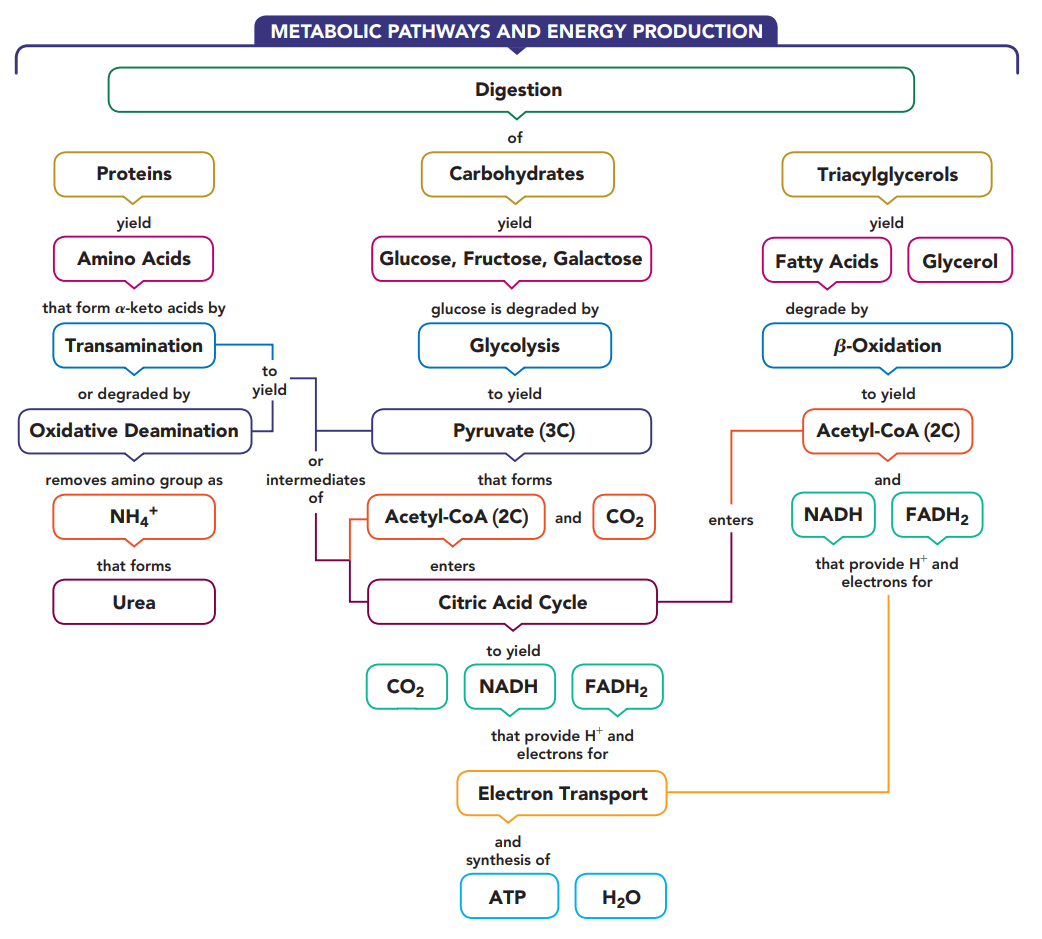
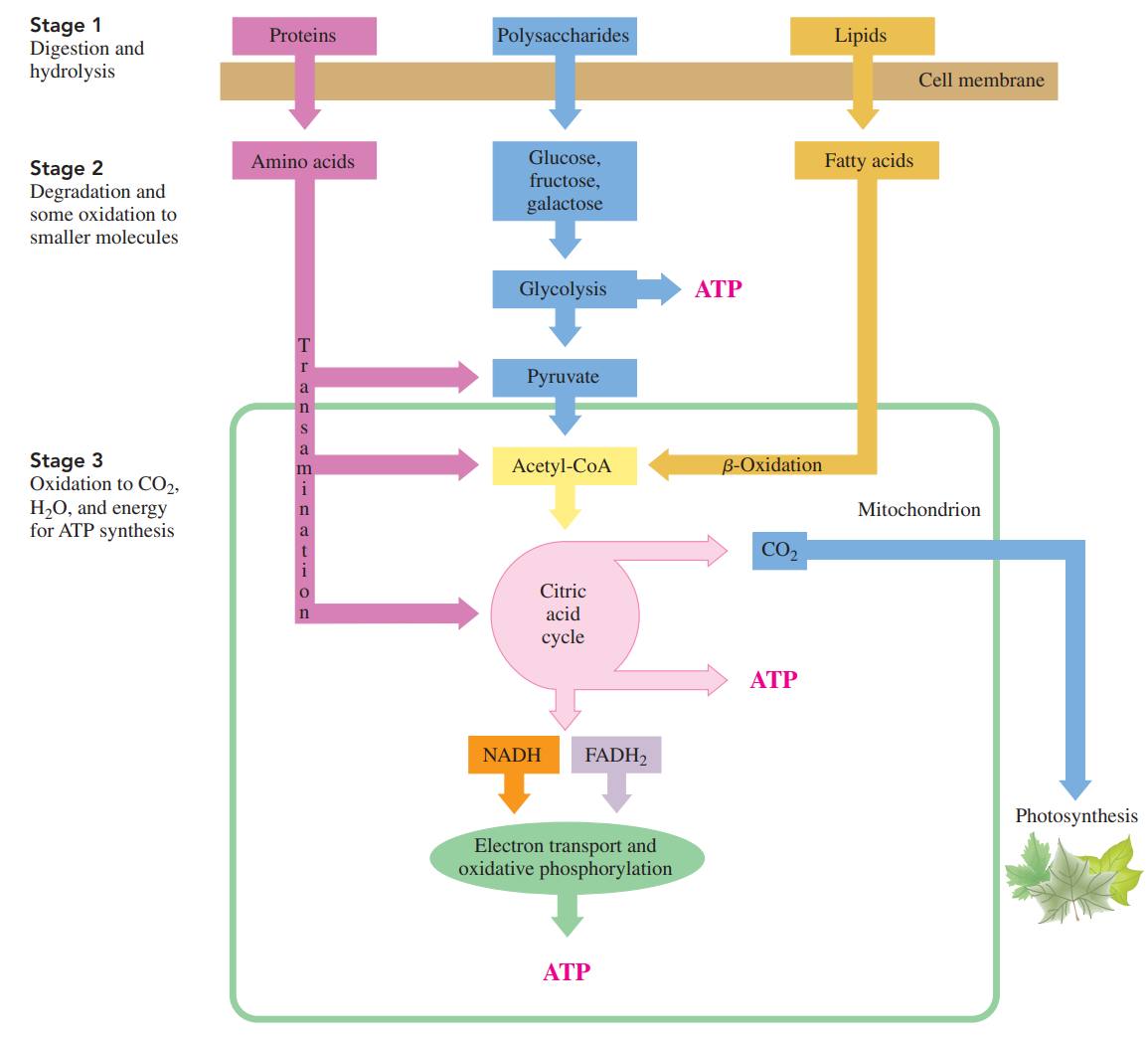
Three Stages of Catabolism
Catabolism begins with the processes of digestion in which enzymes in the digestive tract break down large molecules into smaller ones.
- The polysaccharides break down to monosaccharides, fats break down to glycerol and fatty acids, and the proteins yield amino acids.
- These digestion products diffuse into the bloodstream for transport to cells.
Within the cells, catabolic reactions continue as the digestion products are broken down further to yield two- and three-carbon compounds.
The major production of energy takes place in the mitochondria, as the two-carbon acetyl group is oxidized in the citric acid cycle.
As long as the cells have oxygen, the hydrogen ions and electrons from the reduced coenzymes are transferred to electron transport to synthesize ATP.
18.2: Digestion of Foods
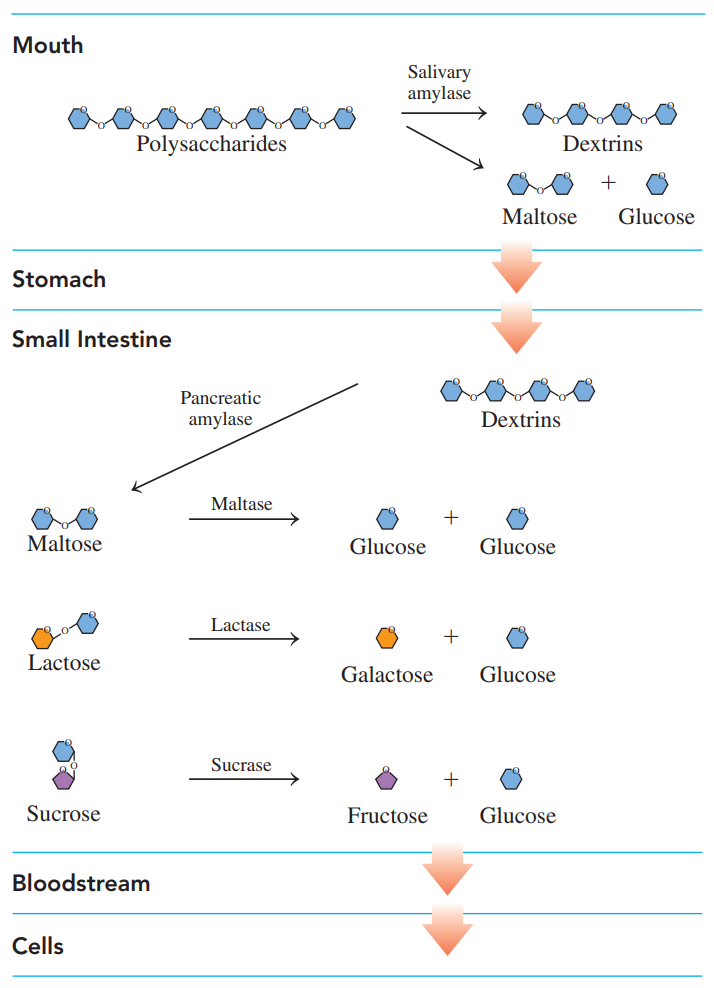
Digestion of Carbohydrates
Enzymes produced in the salivary glands hydrolyze some of the 𝜶-glycosidic bods in amylose and amylopectin, producing maltose, glucose, and dextrins — which contain three to eight glucose units.
After swallowing, the partially digested starches enter the acidic environment of the stomach, where the low pH stops carbohydrate digestion.
In the small intestine, which has a pH of about 8, enzymes produced in the pancreas hydrolyze the remaining dextrins to maltose and glucose.
Then enzymes produced in the mucosal cells that line the small intestine hydrolyze maltose as well as lactose and sucrose.
The resulting monosaccharides are absorbed through the intestinal wall into the bloodstream, which carries them to the liver, where the hexose fructose and galactose are converted to glucose.
Glucose is the primary energy source for muscle contractions, red blood cells, and the brain.
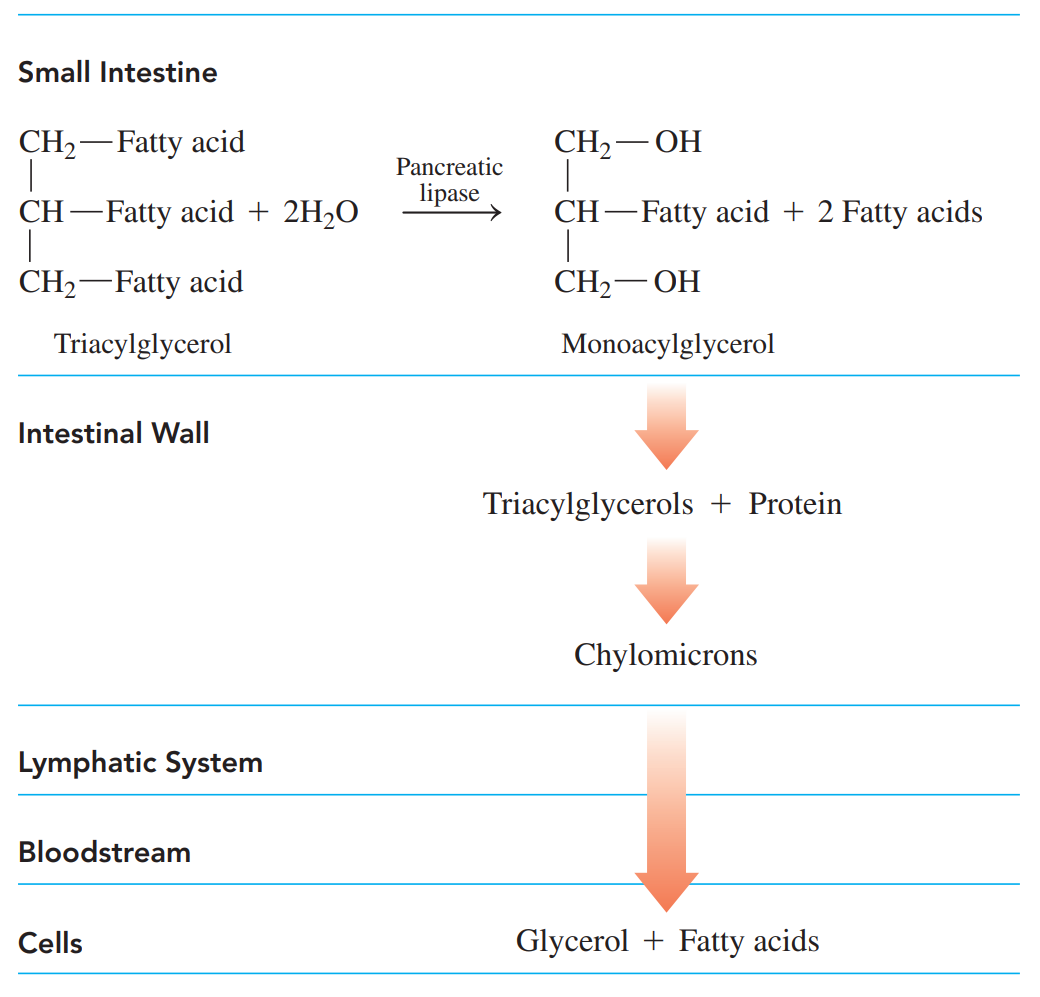
Digestion of Fats
It begins in the small intestine when the hydrophobic fat globules mix with bile salts released from the gallbladder.
Emulsification: A process where the bile salts break the fat globules into micelles.
Enzymes from the pancreas hydrolyze the triacylglycerols to yield monoacylglycerols and fatty acids, which are then absorbed into the intestinal lining where they recombine to form triacylglycerols.
Chylomicrons: The nonpolar compounds are then coated with proteins to form lipoproteins which are more polar and soluble in the aqueous environment of the lymph and bloodstream.
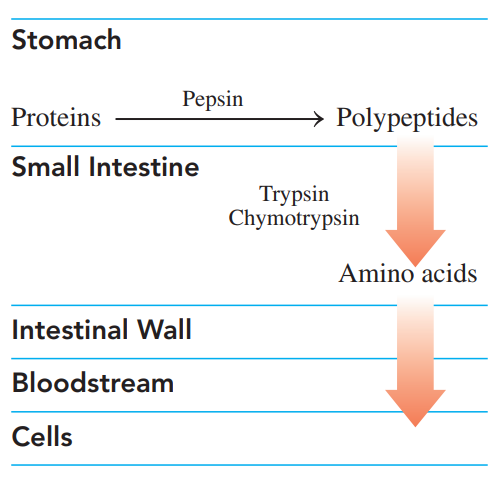
Digestion of Proteins
It begins in the stomach, where hydrochloric acid at pH 2 denatures the proteins and activates enzymes.
Polypeptides move out of the stomach into the small intestine, where trypsin and chymotrypsin complete the hydrolysis of the peptides to amino acids.
The amino acids are absorbed through the intestinal walls into the bloodstream for transport to the cells.
18.3: Coenzymes in Metabolic Pathways
Oxidation: A reaction that involves the loss of hydrogen or electrons by a substance, or an increase in the number of bonds to oxygen.
Reduction: A reaction that involves the gain of hydrogen ions and electrons or a decrease in the number of bonds to oxygen.
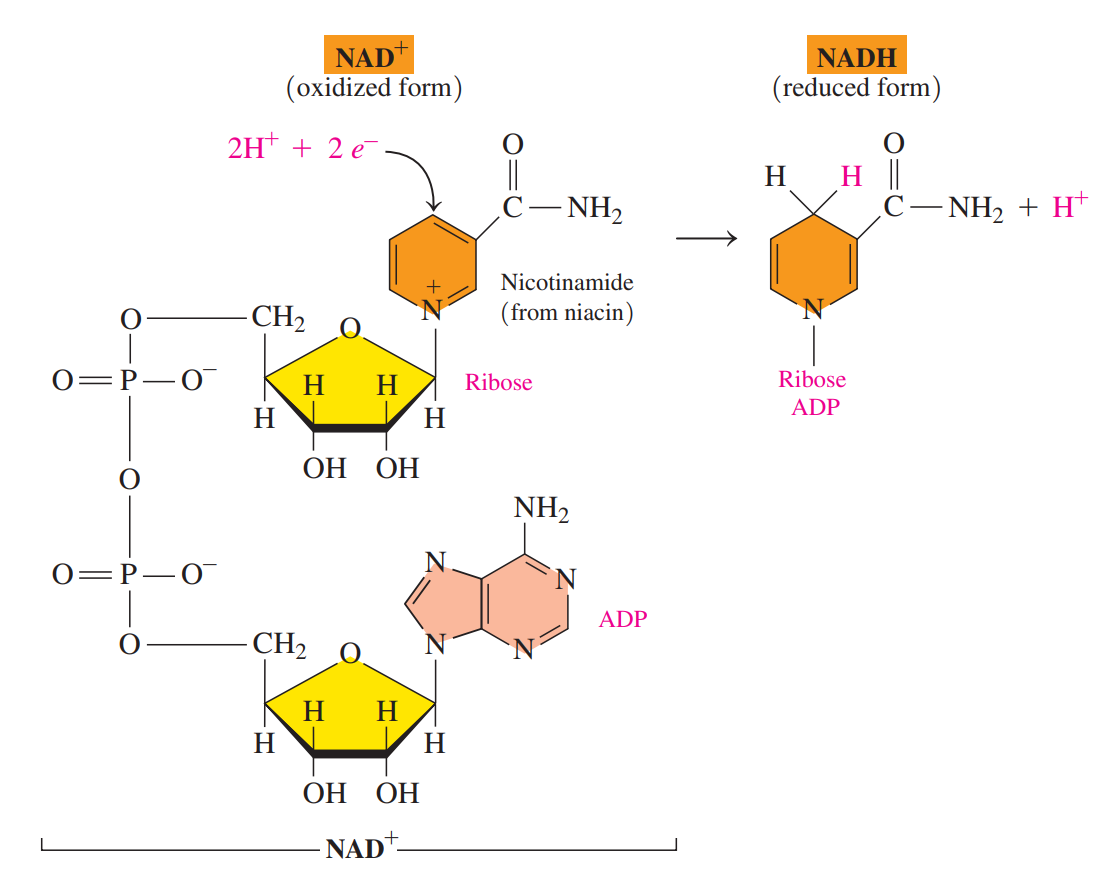
Nicotinamide adenine dinucleotide (NAD+)
An important coenzyme in which the vitamin niacin provides the nicotinamide group, which is bonded to ribose and ADP.
The oxidized NAD+ undergoes reduction when carbon in the nicotinamide ring reacts with 2H, leaving one H+.
The NAD+ coenzyme is required for metabolic reactions that produce carbon–oxygen double bonds.
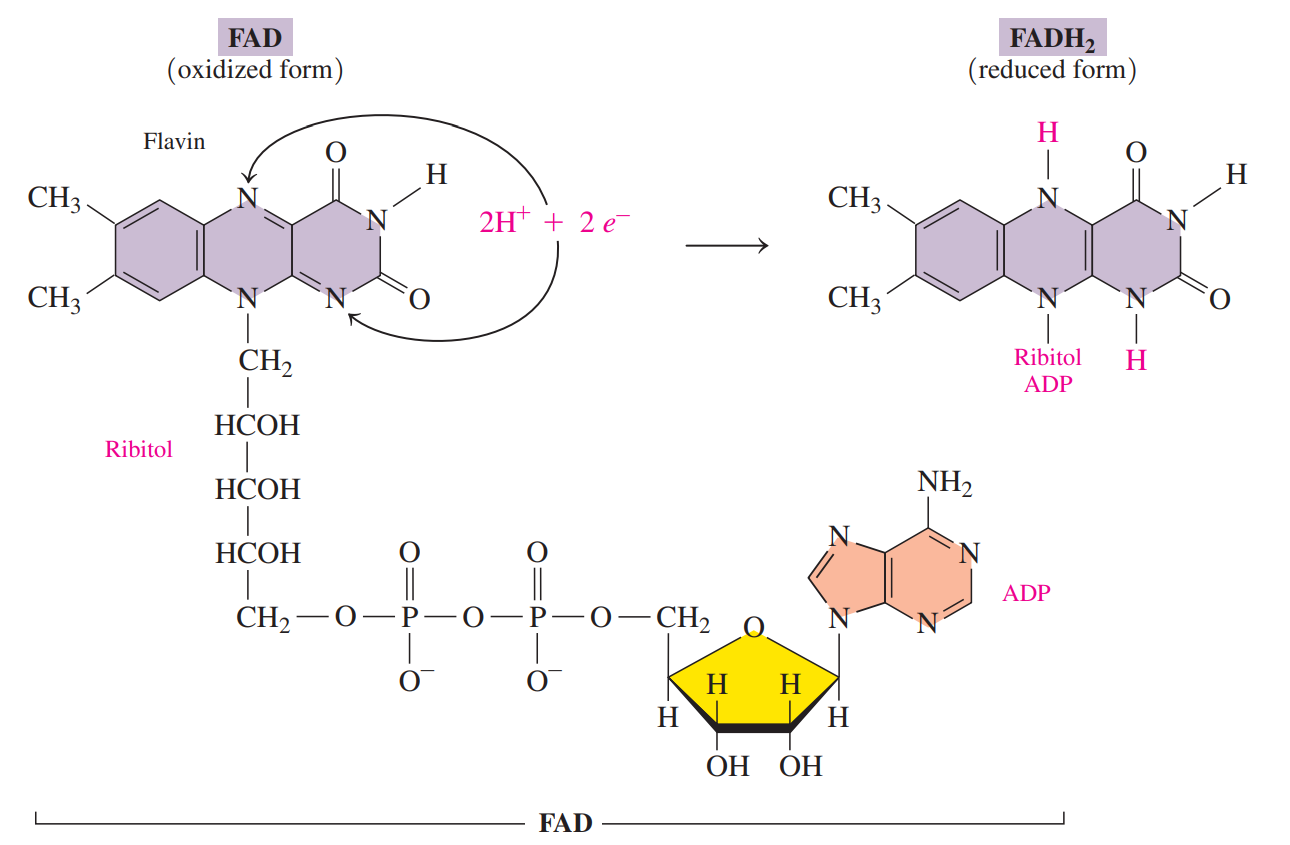
Flavin adenine dinucleotide (FAD)
A coenzyme that contains ADP and riboflavin.
Riboflavin: Also known as Vitamin B2, consists of ribitol and flavin.
The oxidized form of FAD undergoes reduction when the two nitrogen atoms in the flavin part of the FAD coenzyme react with 2H reducing FAD to FADH2.
It is used as a coenzyme when an oxidation reaction converts a carbon–carbon single bond to a carbon–carbon double bond.
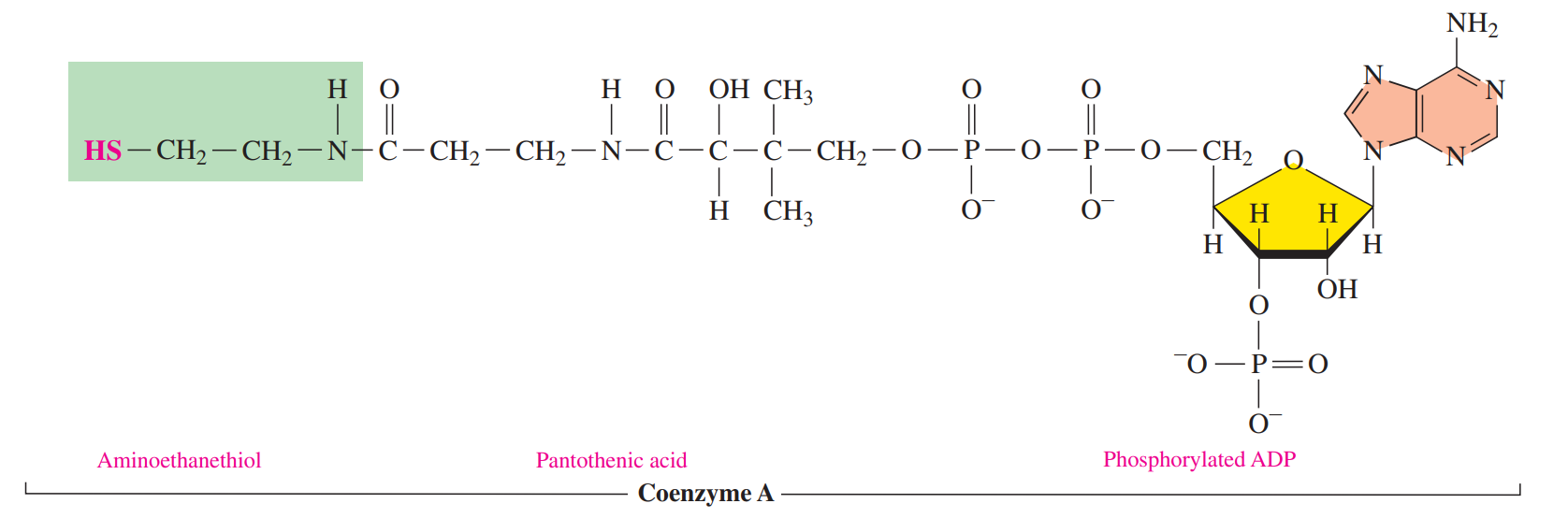
Coenzyme A
Its function is to prepare small acyl groups for reactions with enzymes.
The reactive feature of coenzyme A is the thiol group which bonds to a two-carbon acetyl group to produce the energy-rich thioester acetyl-CoA.
18.4: Glycolysis: Oxidation of Glucose
Glycolysis
A pathway wherein the glucose in the bloodstream enters our cells where it undergoes degradation.
It is an anaerobic process; no oxygen is required.
A six-carbon glucose molecule is broken down to two molecules of three-carbon pyruvate.
All the reactions in glycolysis take place in the cytoplasm of the cell.
Energy-investing phase: The energy is obtained from the hydrolysis of two ATP, which is needed to form sugar phosphates; the first five reactions.
In reactions 4 and 5, a six-carbon sugar phosphate is split to yield two molecules of three-carbon sugar phosphate.
Energy-generating phase: The energy is obtained from the hydrolysis of the energy-rich phosphate compounds and used to synthesize four ATP; the last five reactions (6-10).
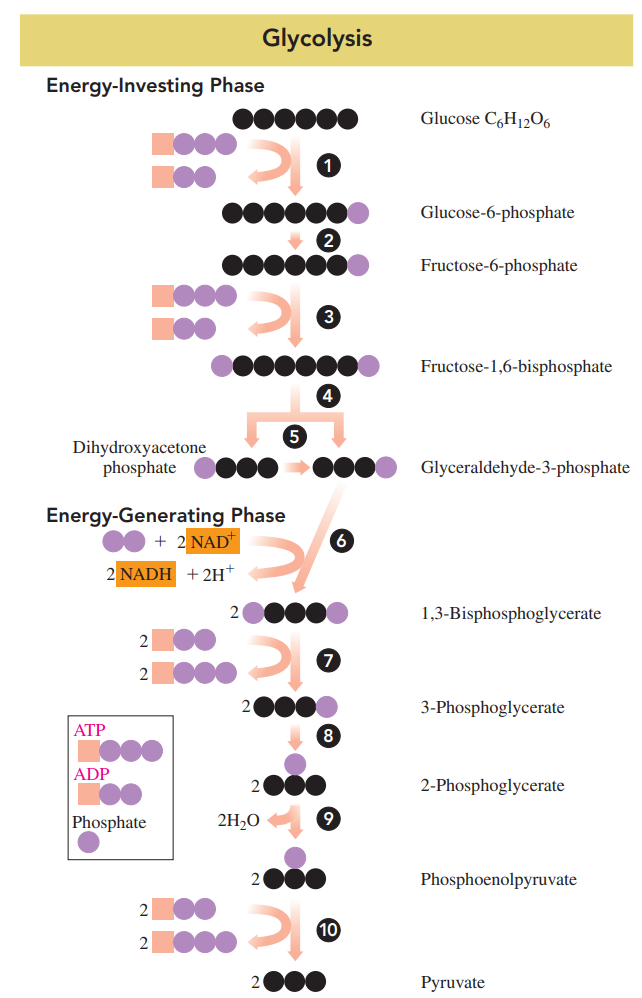
Energy-Investing Reactions 1 to 5
- Reaction 1: Phosphorylation
- In the initial reaction, a phosphate group from ATP is added to glucose to form glucose6-phosphate and ADP.
- Reaction 2: Isomerization
- The glucose-6-phosphate, the aldose from reaction 1, undergoes isomerization to fructose6-phosphate, which is a ketose.
- Reaction 3: Phosphorylation
- The hydrolysis of another ATP provides a second phosphate group, which converts fructose-6-phosphate to fructose-1,6-bisphosphate.
- Reaction 4: Cleavage
- Fructose-1,6-bisphosphate is split into two three-carbon phosphate isomers: dihydroxyacetone phosphate and glyceraldehyde-3-phosphate.
- Reaction 5: Isomerization
- Because dihydroxyacetone phosphate is a ketone, it cannot react further. However, it undergoes isomerization to provide a second molecule of glyceraldehyde-3-phosphate, which can be oxidized.
Energy-Generating Reactions 6 to 10
- Reaction 6: Oxidation and Phosphorylation
- The aldehyde group of each glyceraldehyde-3-phosphate is oxidized to a carboxyl group by the coenzyme NAD+, which is reduced to NADH and H+.
- A phosphate group adds to each of the new carboxyl groups to form two molecules of the high-energy compound, 1,3-bisphosphoglycerate.
- Reaction 7: Phosphate Transfer
- Phosphorylation transfers a phosphate group from each 1,3-bisphosphoglycerate to ADP to produce two molecules of the high-energy compound ATP.
- At this point in glycolysis, two ATP are produced, which balance the two ATP consumed in reactions 1 and 3.
- Reaction 8: Isomerization
- Two 3-phosphoglycerate molecules undergo isomerization, which moves the phosphate group from carbon 3 to carbon 2 yielding two molecules of 2-phosphoglycerate.
- Reaction 9: Dehydration
- Each of the phosphoglycerate molecules undergoes dehydration 1loss of water2 to give two high-energy molecules of phosphoenolpyruvate.
- Reaction 10: Phosphate Transfer
- In a second direct phosphorylation, phosphate groups from two phosphoenolpyruvate are transferred to two ADPs to form two pyruvate and two ATP.
Pathways for Pyruvate
- The pyruvate produced from glucose can now enter pathways that continue to extract energy.
- Aerobic Conditions
- In glycolysis, two ATP were generated when one glucose molecule was converted to two pyruvates.
- Under these conditions, pyruvate moves from the cytoplasm into the mitochondria to be oxidized further.
- In a complex reaction, pyruvate is oxidized, and a carbon atom is removed from pyruvate as CO2.
- The coenzyme NAD+ is reduced during oxidation.
- The resulting two-carbon acetyl compound is attached to CoA, producing acetyl-CoA, an important intermediate in many metabolic pathways
- Anaerobic Conditions
- When we engage in strenuous exercise, the oxygen stored in our muscle cells is quickly depleted.
- Under these conditions, pyruvate remains in the cytoplasm where it is reduced to lactate.
- NAD+ is produced and is used to oxidize more glyceraldehyde3-phosphate in the glycolysis pathway, which produces a small but needed amount of ATP.
18.5: The Citric Acid Cycle
Citric Acid Cycle: A series of reactions connects the intermediate acetyl-CoA from the metabolic pathways in stages 1 and 2 with electron transport and the synthesis of ATP in stage 3.
- It is also known as the tricarboxylic acid (TCA) cycle or the Krebs cycle.
Citric Acid: A tricarboxylic acid, forms in the first reaction.
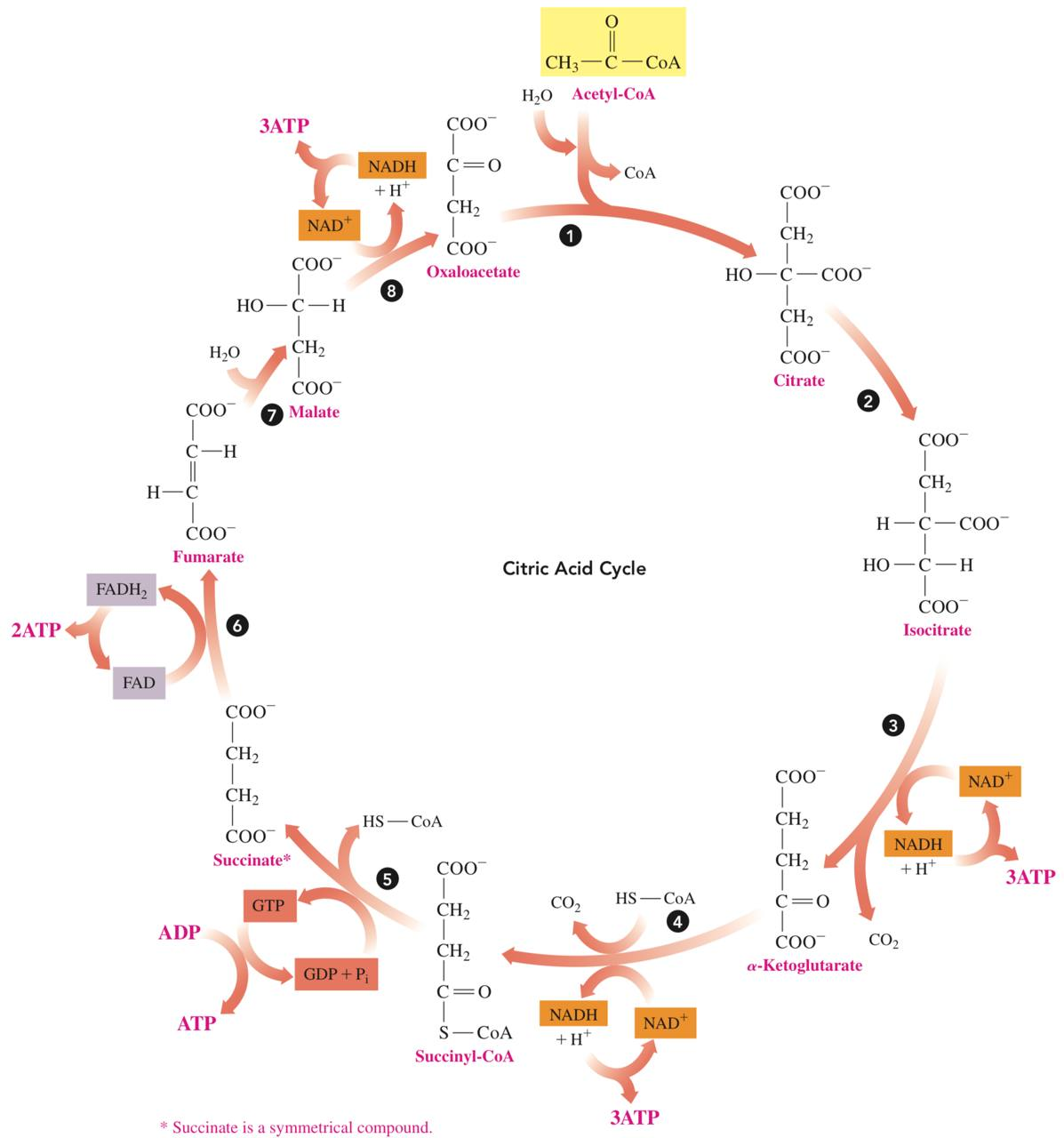
The Cycle
- Reaction 1: Formation of Citrate
- In the first reaction of the citric acid cycle, the acetyl group from acetyl-CoA bonds with oxaloacetate to yield citrate.
- Reaction 2: Isomerization
- The citrate produced in reaction 1 contains a tertiary alcohol group that cannot be oxidized further
- The citrate undergoes isomerization to yield its isomer isocitrate, which provides a secondary alcohol group that can be oxidized in the next reaction.
- Reaction 3: Oxidation and Decarboxylation
- The secondary alcohol group in isocitrate is oxidized to a ketone.
- A decarboxylation converts a carboxylate group to a CO2 molecule producing 𝜶-ketoglutarate.
- The oxidation reaction also produces hydrogen ions and electrons that reduce NAD+ to NADH and H+.
- This reduced coenzyme NADH will be important in the energy-producing reactions we will discuss in electron transport
- Reaction 4: Oxidation and Decarboxylation
- 𝜶-ketoglutarate undergoes oxidation and decarboxylation to produce a four-carbon group that combines with CoA to form succinyl-CoA
- Reaction 5: Hydrolysis
- Succinyl-CoA undergoes hydrolysis to succinate and CoA. The energy released is used to add a phosphate group to GDP which yields GTP.
- Reaction 6: Oxidation
- Hydrogen is removed from each of two carbon atoms in succinate, which produces fumarate, a compound with a trans double bond.
- Reaction 7: Hydration
- Hydration adds water to the double bond of fumarate to yield malate, which is a secondary alcohol.
- Reaction 8: Oxidation
- The last step of the citric acid cycle, the secondary alcohol group in malate is oxidized to oxaloacetate, which has a ketone group.
18.6: Electron Transport and Oxidative Phosphorylation
In electron transport, hydrogen ions and electrons from NADH and FADH2 are passed from one electron carrier to the next until they combine with oxygen to form H2O.
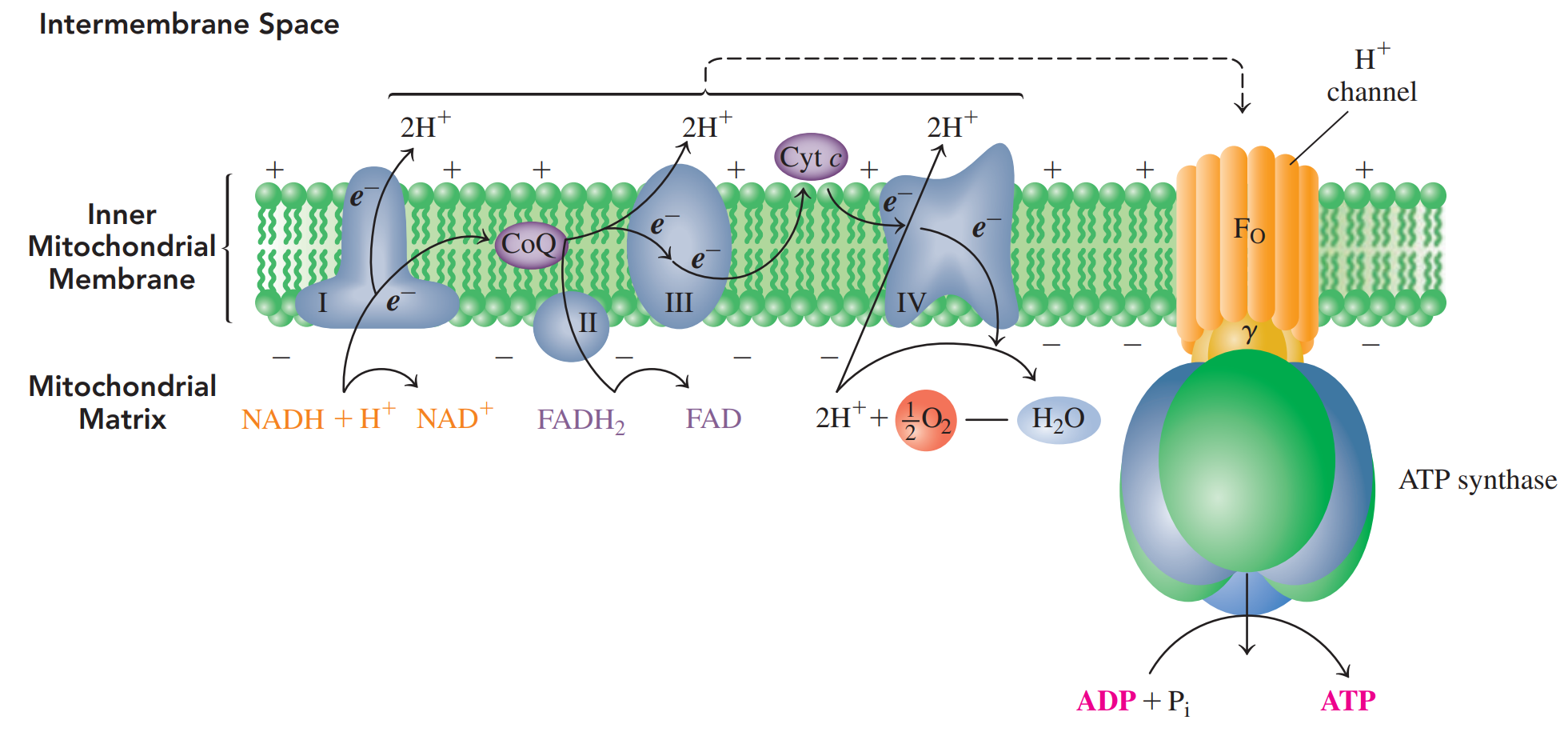
Oxidative phosphorylation: The energy released during electron transport is used to synthesize ATP from ADP and Pi.
- Chemiosmotic model: Links the energy from electron transport to a H+ gradient that drives the synthesis of ATP.
ATP Synthesis: An enzyme complex that uses the energy released by H+ ions returning to the matrix to synthesize ATP from ADP and Pi .
ATP from Glycolysis
- In glycolysis, the oxidation of glucose stores energy in two NADH molecules as well as two ATP from direct phosphate transfer.
- However, glycolysis occurs in the cytoplasm, and the NADH produced cannot pass through the mitochondrial membrane.
ATP from the Oxidation of Two Pyruvate
- Under aerobic conditions, pyruvate enters the mitochondria, where it is oxidized to give acetyl-CoA, CO2, and NADH.
- Because glucose yields two pyruvates, two NADH enter electron transport, where the oxidation of two pyruvate leads to the production of six ATP.
ATP from the Citric Acid Cycle: One turn of the citric acid cycle produces two CO2, three NADH, one FADH2, and one ATP by direct phosphate transfer.
ATP from the Complete Oxidation of Glucose: The total ATP for the complete oxidation of glucose is calculated by combining the ATP produced from glycolysis, the oxidation of pyruvate, and the citric acid cycle.
18.7: Oxidation of Fatty Acids
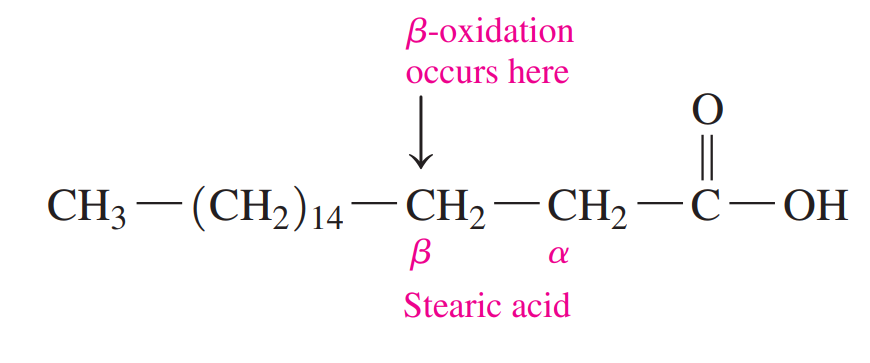
A large amount of energy is obtained when fatty acids undergo oxidation in the mitochondria to yield acetyl-CoA.
Beta-oxidation: This is where fatty acids undergo the removal of two-carbon segments, one at a time, from the carboxyl end.
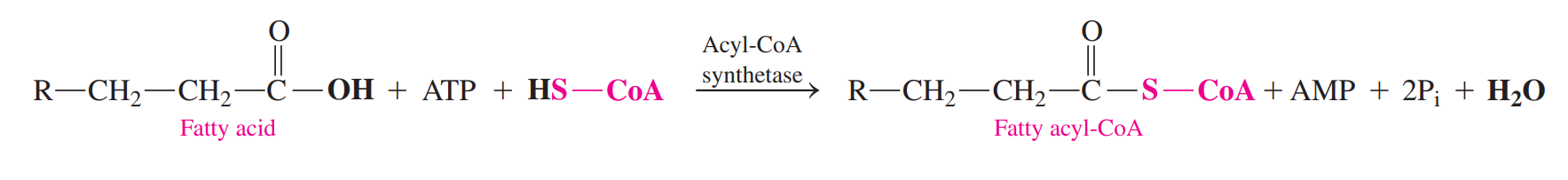
Fatty Acid Activation: It combines fatty acid with coenzyme A to yield fatty acyl-CoA.
The energy for the activation is obtained from the hydrolysis of ATP to give AMP and two inorganic phosphates.
Ketone Bodies: The products of ketogenesis: are acetoacetate, 𝜷-hydroxybutyrate, and acetone.
Ketosis: A condition of the accumulation of ketone bodies; which occurs in severe diabetes, diets high in fat and low in carbohydrates, alcoholism, and starvation.
18.8: Degradation of Amino Acids
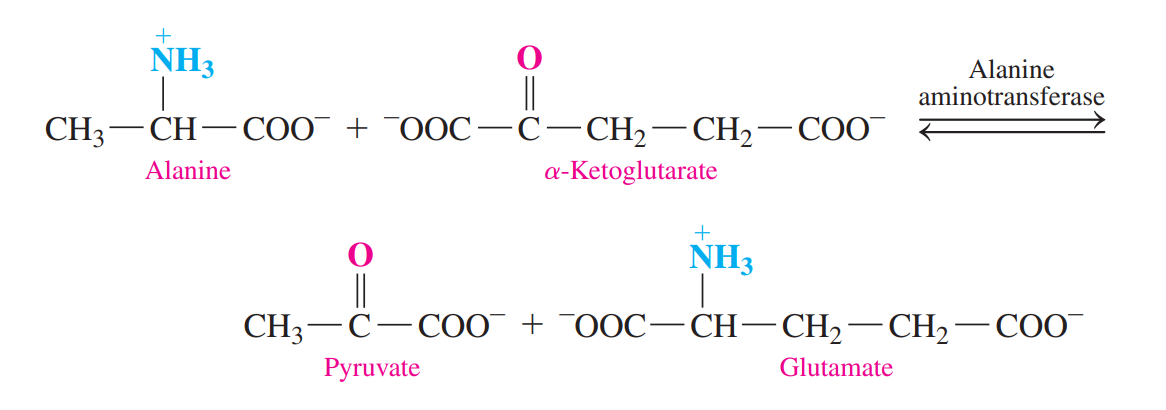
Transamination
An 𝜶-amino group is transferred from an amino acid to an a-keto acid, usually a-ketoglutarate.
A new amino acid and a new 𝜶-keto acid.
Oxidative Deamination: The ammonium group in glutamate is removed as an ammonium ion.
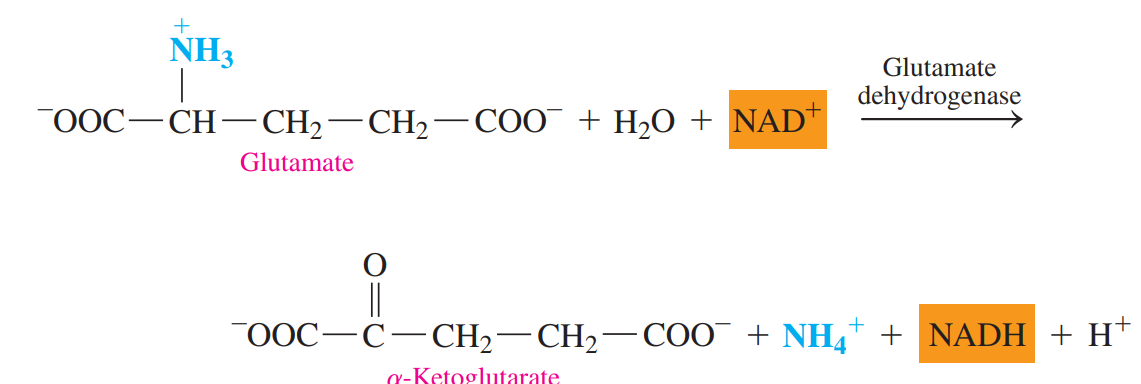
Urea Cycle: A series of reactions that detoxifies ammonium ions by forming urea.
The ammonium ion, which is the end product of amino acid degradation, is toxic if it is allowed to accumulate.