Lecture 2 not finished (copy)
1/21
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
What is an arene?
aromatic hydrocarbons
What is an aryl group?
aromatic hydrocarbon with one hydrogen atom removed
what is an electrophile
electron - loving or electron deficient species
What is a phenyl group?
Benzene ring with 1 hydrogen atom removed
What can an electrophile do?
React with a benzene ring and substitute for one of the hydrogen atoms
What reaction does benzene undergo?
Substitution reactions
What do electrophiles attack the π system of benzene form?
A delocalised non-aromatic carbocation
What happens in the first step of electrophilic substitution?
The electrophile takes 2 electrons from the system to form a σ bond in 1 of the carbon atoms
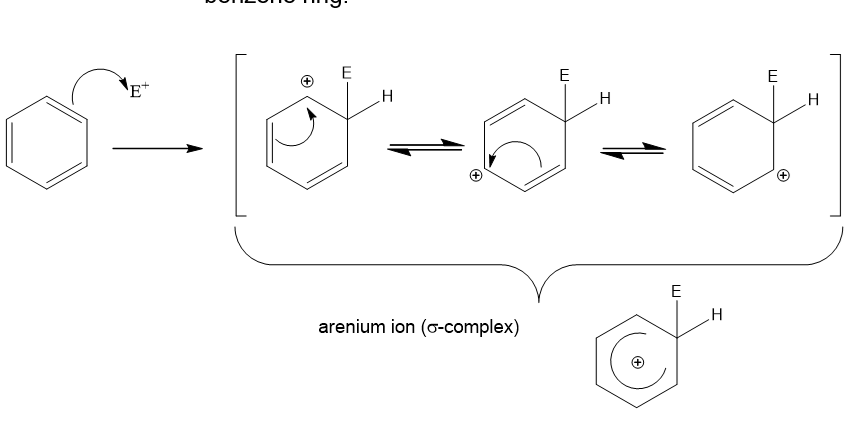
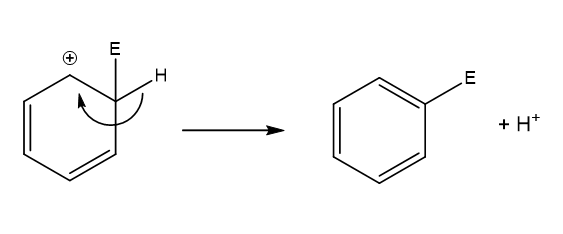
What happens in step 2 of electrophilic substitution?
The arenium ion loses a proton from the carbon with the electrophile.
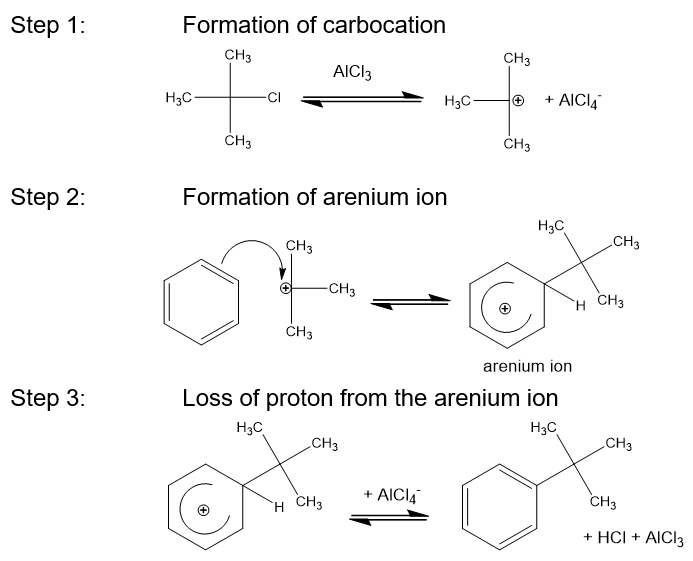
What is the carbocation generated by in Friedel-Crafts alkylation?
AlCl3
In FC alkylation what are the 3 steps?
Formation of carbocation
Formation of arenium ion
Loss of proton from the arenium ion
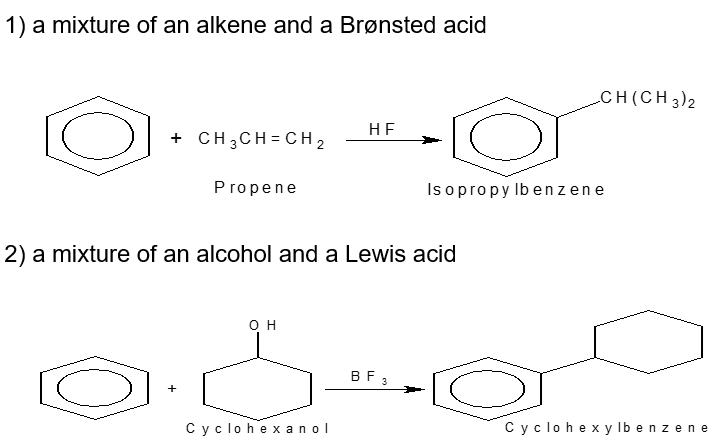
What are examples of Friedel-Crafts alkylation(2)?
A mixture of an alkene and Bronsted acid
A mixture of an alcohol and Lewis acid
What happens to aromatic alkyl halides in Friedel-Crafts alkylation reaction
They do not react
When does the Friedel-Crafts alkylation reaction not take place(2)?
If rings contain electron withdrawing substituents
N group present
What is rearranged in Friedel-Crafts alkylation reaction?
Carbocation
Which multiple reactions often take place in Friedel-Crafts alkylation reaction?
Substitution
What does the primary alkyl halide-AlCl3 complex undergo?
A proton shift rearrangement to generate a more stable 2o carbocation
What happens in the halogenation of benzene?
In the presence of a Lewis acid, benzene reacts with Br or Cl to produce a halobenzene
What are examples of halobenzene?
Bromobenzene or chlorobenzene
What happens in the nitration of benzene?
Benzene reacts slowly with hot HNO3 to form nitrobenzene
What can make the nitration of benzene faster?
If a mixture of HNO3 and H2SO4 is used
How does H2SO4 increase the rate of reaction?
By increasing the concentration of NO2+