orgo chem carboxylic acids and esters
1/72
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
73 Terms
How do we name the parent chain that has a carboxylic acid?
By using the suffix -oic acid
What is a dicarboxylic acid?
An acid that contains two carboxyl groups at each end of the parent chain
What are poly functional acids?
Acids that contain one or more additional functional groups besides the carboxyl group
Are carboxlyic acids the most polar or nonpolar compound?
Polar
How are carboxylic acids made?
By the oxidation of a primary alcohol and aldehyde
What is a carboxylate ion?
A negative ion produced when a carb acid loses one or more acidic hydrogen ions
How can the solubility of a carb acid be greatly increased?
By converting them to a carboxylic acid salt
Esters are derivatives of what? What do they replace?
Carboxylic acids. Esters replace the -OH in COOH with -OR, COOR.
The condensation reaction of a carb acid with an alcohol by using a strong acid is called what?
Esterification
Low molecular mass esters are soluble in water because?
Esters can hydrogen bond with water, so they are soluble as long as their molecular weight is small
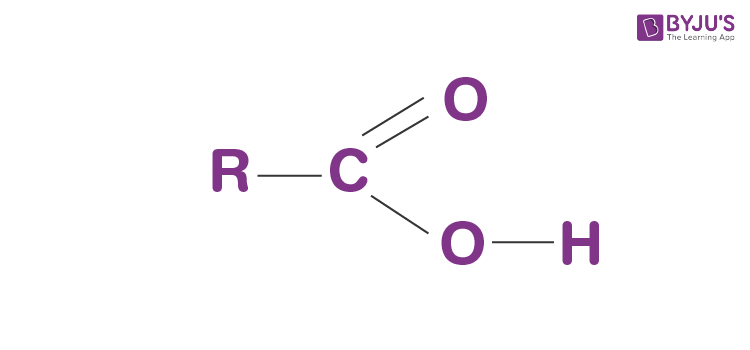
What functional group is this?
Carboxyl group
Can carboxyl groups be a part of ring structures?
No, they can only be attached by way of making the ring structure a substituent.
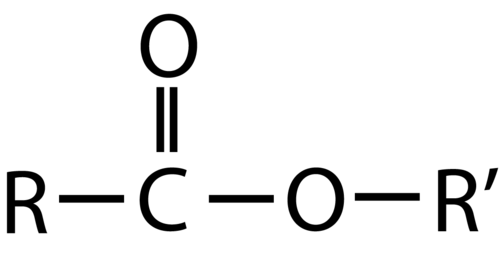
What functional group is this? Is this a carboxylic acid derivative?
Ester, yes it is
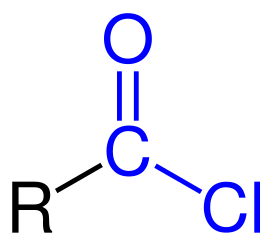
What functional group is this? Is this a carboxylic acid derivative?
Acid chloride, yes it is
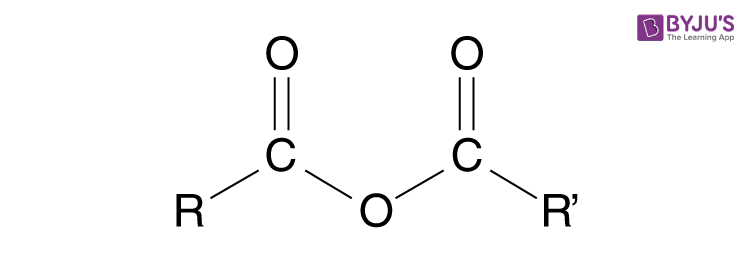
What functional group is this? Is this a carboxylic acid derivative?
Acid anhydride, yes it is
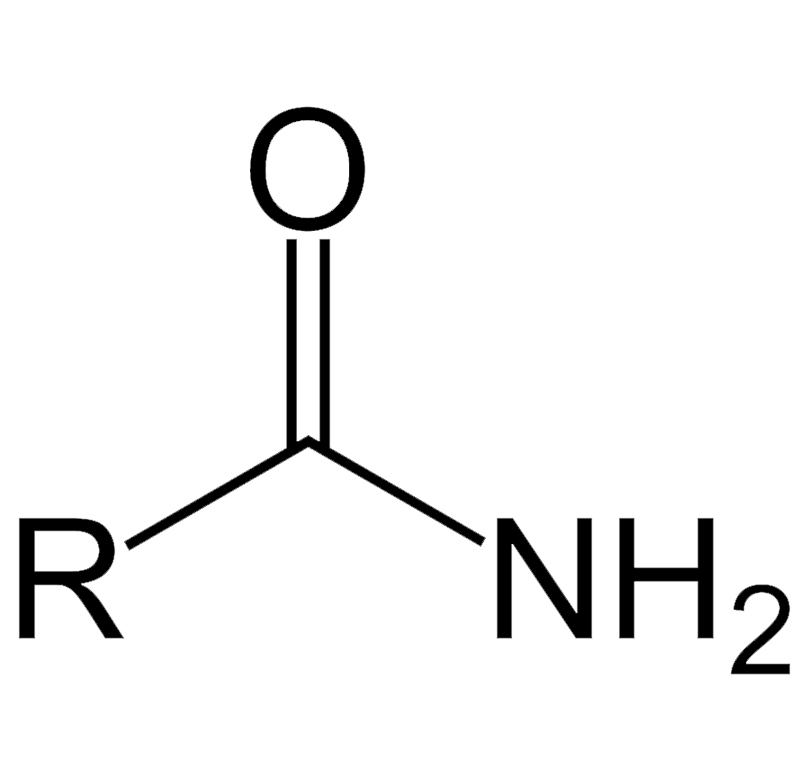
What functional group is this? Is this a carboxylic acid derivative?
Amide, yes it is
What is the acyl group?
The portion of the molecule that remains when Z group has been removed
What does “Z” mean when it comes to acyl groups?
The Z group is the portion of molecule that describes the differences in molecules, such as in Carboxylic acids, the -OH is the Z group, in Esters the -OR is the Z group, in Acid chloride the -Cl is the Z group, etc…
What is the difference between carbonyl compounds and acyl compounds?
In a carbonyl compound, the carbon is attached to a hydrogen or another atom. In an acyl compound the carbon is attached to specifically an oxygen, nitrogen, or halogen group.
Which functional groups are carbonyl compounds?
aldehydes and ketones
Which functional groups are acyl groups?
carboxylic acids and their derivatives
For common name, what suffix do we use when naming a carboxylic acid with one carbon?
-form
For common name, what suffix do we use when naming a carboxylic acid with two carbons?
-acet
For common name, what suffix do we use when naming a carboxylic acid with three carbons?
-propion
For common name, what suffix do we use when naming a carboxylic acid with four carbons?
-butyr
For common name, what suffix do we use when naming a carboxylic acid with five carbons?
-valer
For common name, what suffix do we use when naming a carboxylic acid with six carbons?
-capro
For common name, what greek letter do we use for a carboxylic acid if a substituent is on the second carbon?
a
For common name, what greek letter do we use for a carboxylic acid if a substituent is on the third carbon?
B
For common name, what greek letter do we use for a carboxylic acid if a substituent is on the fourth carbon?
y
For common name, what greek letter do we use for a carboxylic acid if a substituent is on the fifth carbon?
S
What is the pneumonic for common name prefixes for carboxylic acids?
Frogs are polite, being very courteous (Formic, acet, propin, butyr, valeric, capro)
For common name, what suffix do we use when naming a dicarboxylic acid with two carbons?
oxal-
For common name, what suffix do we use when naming a dicarboxylic acid with three carbons?
malon-
For common name, what suffix do we use when naming a dicarboxylic acid with four carbons?
-succin
For common name, what suffix do we use when naming a dicarboxylic acid with five carbons?
glutar-
For common name, what suffix do we use when naming a dicarboxylic acid with six carbons?
adip-
For common name, what suffix do we use when naming a dicarboxylic acid with seven carbons?
pimel-
Do we also use greek letters when using common name for dicarboxylic acids?
Yes
Is there a dicarboxylic acid that has one carbon?
No there isn’t
What is the pneumonic for dicarboxylic acids?
Oh my, such good apple pie (Oxal, malonic, succin, glutar, adip, pimel-)
What is an unsaturated acid?
A polyfunctional acid that contains a double bond and has a wide variety of uses
What is a hydroxy acid?
An acid that contains a hydroxyl group
What is a keto acid?
Acid that has a ketone and carboxyl group
Carboxylic acids are soluble when they have how many carbons?
Less than five
Carboxylic acids boiling point increases as?
The number of carbons increase
What is the tier list of compounds when it comes to boiling point? (highest to lowest)
Acids → alcohol → aldehydes → alkanes
Can carboxylic acids hydrogen bond to themselves?
Yes
Ionization
Occurs in aqueous solution, acid → carboxylate ion (COO-)+ H3O+
neutralization
reaction with a base (NaOH), acid → carboxylate salt (CONa+) + H2O
decarboxylation
A carbon dioxide molecule is produced, acid → CO2 + smaller organic molecule
Is a carboxylate ion a weak or strong acid?
Weak acid
How do we name a carboxylate ion?
-ic acid turns into -ate
How do we name an ester?
Name the alkyl part first (the group extending from the second O) and then name the acyl group, finally add the suffix -oate
What makes the smell/odor of raspberry?
Isobutyl methanoate - R/IBM
What makes the smell/odor of pear?
Propyl ethanoate - P/PE
What makes the smell/odor of banana
Pentyl ethanoate - B/PE
What makes the smell/odor of orange?
octyl ethanoate - O/OE
What makes the smell/odor of apricot?
pentyl propionate - A/PP
What makes the smell/odor of apple?
methyl butanoate - A/MB
What makes the smell/odor of pineapple?
ethyl butanoate - P/EB
What else do esters make?
pheromones
What is the alarm pheromone in bees?
Isoamyl acetate AB/ISA
What are commonly used esters?
Benzocaine - used as a local anesthesia
Aspirin - used as an analgesic
Oil of wintergreen - used in rubs and creams for muscle soreness
Can esters hydrogen bond in water?
Yes
Can esters hydrogen bond to themselves?
No
What is the solubility limit of esters?
The solubility limit is fewer than 5 carbons
Esters are completely soluble with how many carbons?
1-2 carbons
Esters are slightly soluble with how many carbons?
3-5 carbons
Is an esters boiling point higher or lower compared to aldehyde or alcohol with a similar number of carbons?
Lower
What is the tier list of boiling point based on lowest to highest?
Ether, ester, aldehyde, alcohol, acid
What is the reverse reaction of condensation? What products are made? Is a strong acid or base used?
Acidic hydrolysis, COOR + H2O → COOH + R-OH, a strong acid
What is hydrolysis of an ester under basic conditions, and what product is made?
Ester saponification, COOR + NaOH (strong base) → COONa+ (carboxylate salt) + R-OH