theme 1 : Smooth Muscle Cells structure CML
1/83
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
84 Terms
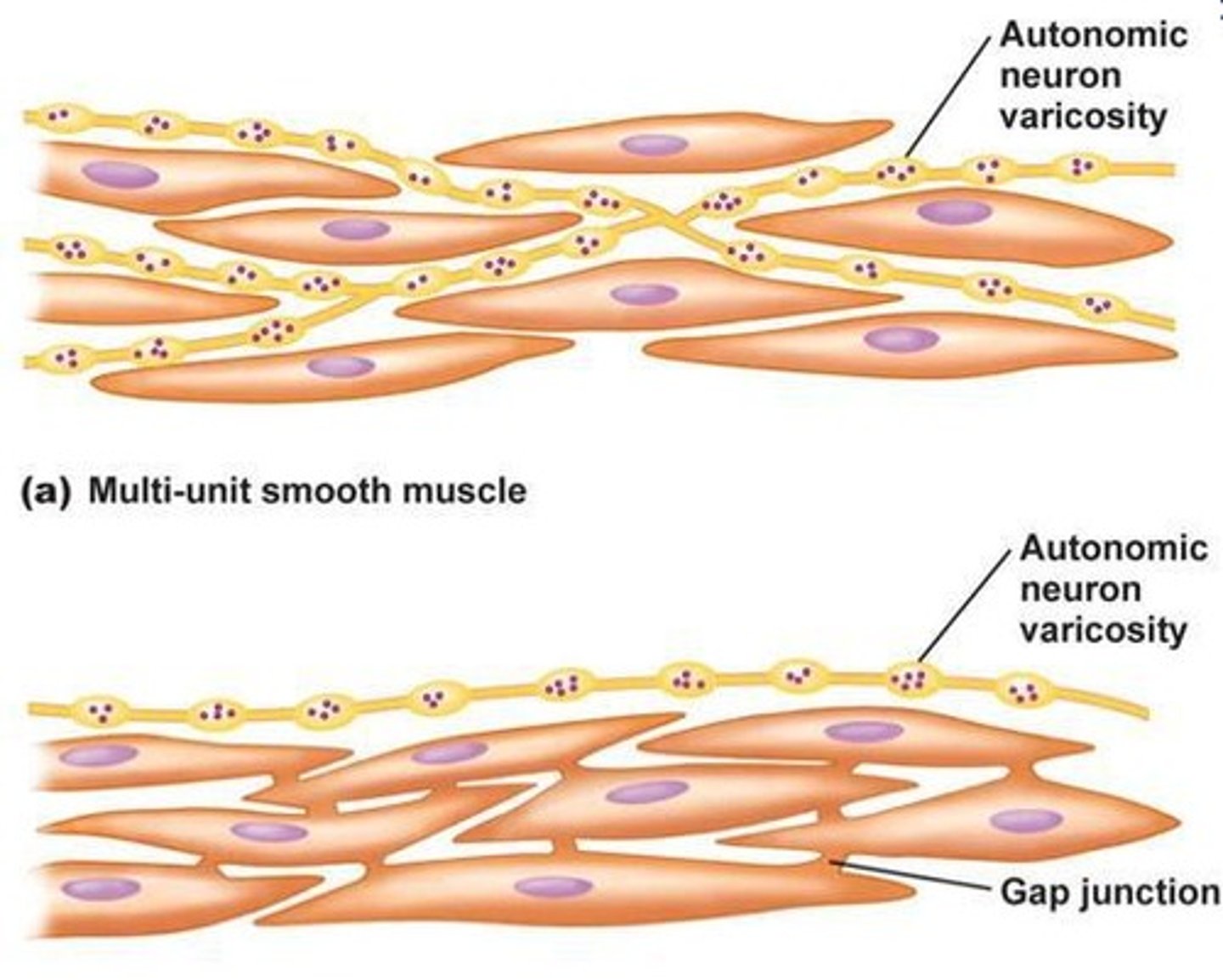
What are the two types of smooth muscle cells?
Multi-unit smooth muscles → autonomic neuron varicosity surrounding each CML (so independent)
each cell must receive a signal
noradrenaline from orthosympathetic nervous system
acetylcholine from parasympathetic system
unitary smooth muscles → gap junctions between CML so they are all connected
one activation activates all of them
what does a sarcomere look like
z = point d'ancrage de filament d'actine
M = support filament eppais mysosine
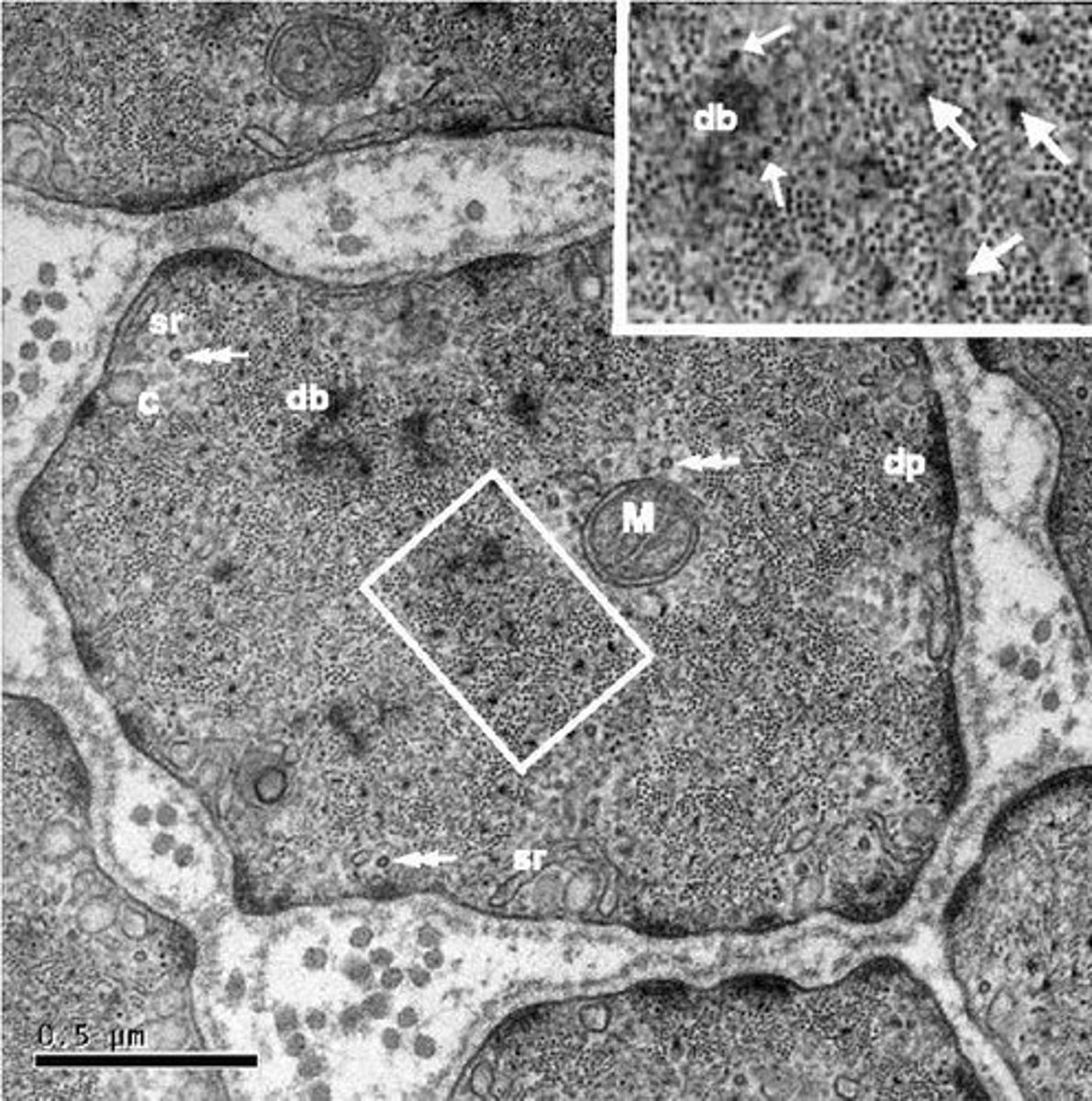
what is observed in a CML under micrscopy
mitochondria
Dense body db → corps dense aux e- → zone d'ancrage des filaments fins active
dense plate dp → plaque dense aux e-
sarcoplasmique reticulum
caveaola
microtubules
What is the role of dense bodies in smooth muscle cells?
Dense bodies anchor thin filaments in smooth muscle cells and the thick filament stays in between the 2 thin filaments held by dense body
what are the 6 isoforms of actin
contractile :
- skeletal alpha actin
- cardiac alpha actin
- smooth muscle alpha actin
- smooth intestinal gamma actin
cytoskeletal :
- cytosolic gamma actin
- beta actin (in all cells)
what are the contractile filaments of CML
heavy filament : myosin filament + myosin heads
light filament : smooth muscle alpha actin + tropomyosin + caldesmon + calponin
role of tropomyosin
stabilise actin filament and can be homo/heterodimer
role of caldesmon and calponin
inhibitor effector and stabilises actin filament
when bound to calcium and therefore phosphorylated it becomes inactive
what are the two important myosin light chains
MLC20 and MLC17 = central point of contraction of CML depending on phosphorylation
what are the two states myosin light chains (MLC) in smooth muscle contraction?
Inactivated : dephosphorylated and folded conformation (MLC20) and cant bind to actin filament
Activated : MLC20 phosphorylated and becomes linear to bond w/ actin filament -> contraction

how is the phosphorylation of MLC induced
Cytosolic Ca2+ bind with calmodulin
MLC20 interacts w/ Ca-CaM + ATP
MLC20 is phosphorylated -> dimerisation -> contraction
how are the contractile units organised
Functional units (db) = filament fin + eppais qui permet le déplacement physique entre 2 corps dense ou 1 corps dense et 1 corps d'attachement
Plaque d'attachement = filament fin s'attache ici
intermediate filaments = relié les corps dense et permet de maintenir les corps dense dans l'éspace
what occurs to the contractile units during a contraction
the functional units come closer to each-other
contraction de toutes les sense
what are the main regulations of excitation-contraction coupling
local/paracrine
hormonal via blood with through the vasovasorum
nervous -> ortho(maj)/parasympathetic
What are the two types of smooth muscle activity patterns?
Tonic smooth muscles (sustained contraction) and phasic smooth muscles (rhythmic contraction).
what are the mechanisms are involved in excitation-contraction coupling
stimulating ligand that activates a 7 domaine TM coupled to a G protein and RTK enzymatic receptor
voltage dependant channel
stretch activated channel
what are the two types of PA in CML
slow depolarisation waves : parallel PA rapid after passing threshold
spike potential : spikes of PA
PA in CML of Iris arteriole
membrane potentiel = -40 mv
during each PA the diametre of the blood vessel decreases -> contraction
PA of CML in basilar artery
membrane potentiel = -40mv
during each PA diametre increases -> relaxation
what ions enter and leave during a PA
initail depolarisation to reach threshold : exit of Cl-1
once threshold passed theres a depolarisation : calcium channels voltage dependent open and let the entry of calcium
repolarisation : potassium channels are responsible for the exit of K+
membrane potentiel of a CML
-50mv
what are the calcium channels involved in Ca entry
L and T type channels
activity of potassium channels during hyper/depolarisation
Ca channels voltage dependent closed -> K+ channels open and exit CML -> hyperpolarisation -> vasodilation
Ca channels voltage dependent open -> high + charge inside cell -> K+ channels closed -> no K+ exit -> depolarisation -> vasoconstriction
role of potassium channels
maintain resting potential
when are chlore channels activated ?
local increase of intracellular calcium
increase in cell volume
activity of chlore channels during hyper and depolarisation
Ca voltage dependent channels closed -> Cl channels closed -> (-) charge accumulation -> hyperpolarisation -> vasodilation
Ca voltage dependent channels open -> Cl channels open -> exit of Cl- and increases + charge inside cell -> threshold reached -> depolarisation -> vasoconstriction
What are ionotropic receptors?
Receptors that mediate fast synaptic transmission by allowing ions to flow through their channels.
how does noradrenaline/ATP and purinergic(ATP) ionotropic receptors P2X increase intracellular Ca
co-liberation of ATP and noradrenaline by a nerve cell -> ATP binds to purinergic receptors that allow entry of Ca and Na -> purinergic Ca transient -> depolarisation -> activates voltage dependent calcium channels -> increase of intracellular Ca
how can mechanical stress depolarise the ell
mechanical stress -> stretch dependent cation channels SAC are activated -> entry of Ca and Na -> depolarisation
what is the cellular signalling process when mechanical stress activates stretch dependent cation channels
mechanical stress will activate the SAC -> opening of the channel -> entry of Ca into the CML -> Ca-CaM -> MLC phosphorylation -> contraction
What is calcium-induced calcium release (CICR) in smooth muscle cells?
A mechanism where calcium influx triggers further calcium release from the sarcoplasmic reticulum.
Calcium Induced Calcium Release CICR works how ?
L type Ca channels/Ionotropic receptora and SAC are activated -> entry of Ca -> Ca inds to RYR2/3-> stocked Ca leaves sarcoplasmic reticulum SR (-> leaved cell thanks to ATP and enters SR by Ca ATPase SERCA)
do CML have a lot of Ca reserve in sarcoplasmic reticulum compared to CMStrié
no they have less Ca stockage
What is pharmacomechanical coupling?
A process where chemical signals (like hormones) induce muscle contraction through calcium signaling without direct electrical stimulation.
What is L'IP3-induced calcium release?
A pathway where inositol trisphosphate (IP3) stimulates the release of calcium from the endoplasmic reticulum
how does IP3 induce calcium release
ANGII binds to RCPG -> PLC activated -> acts on PIP2 -> DAG + IP3 -> IP3 binds to RIP3 on sarcoplasmic reticulum membrane -> Ca release
what protein g is involved in ANGII recognition and PLC activation
Gq11
What is the function of IP3 in smooth muscle cells?
It induces calcium release from the sarcoplasmic reticulum as a second messenger
what are the main store operated channels SOC and what are they activated by
TRPC 1,4 and 5
activated by the decrease of Ca in SR
What is capacitative calcium entry?
A process by which calcium enters the cell after depletion of calcium stores in the endoplasmic reticulum carried by TRPC 1,4,5 (SOC)
what are main second messenger operated channel SMOC/ROC and what are they operated by
TRPC 3,6 and 7
activated par DAG
how do SOC (TRPC 1,4,5) increase Ca entry into cytoplasm
when SR is depleted from Ca -> oligomerisation of STIM1/2 -> STIM and TRPC interact -> Ca entry into cytoplasm
how do SMOC (TRPC 3,6,7) increase Ca entry into cytoplasm
DAG (produced with IP3 from activity of PLC on PIP2) binds to TRP 1,4,5 -> SOC opens -> Ca entry
what is kinase that phosphorylates MLC to induce contraction
MLCK
how is MLCK (kinase for MLC) activated
when Ca-CaM complex binds to MLCK
what de-phosphorylates MLC
MLCP = MLC phosphatase
what happens to the contractile force and intracellular calcium variation in mous aorta after stimulation
stimulation -> elevation of Ca -> then decrease of intracellular Ca which continues to decrease
stimulation -> increase of contractile force -> plateau (even when Ca decreases) -> slight increase when Ca continues to decrease
if intracellular Ca continues to decrease but contractile force increases (slightly) what does it mean
Ca induces contraction but there is a contractile apparatus sensitivity to calcium
what happens to contraction when agonist noradenaline is added
vessel permeability increases so increased entry of Ca
more sensitive to Ca -> increased contractile response
what mechanism is involved with contractile apparatus sensitivity to Ca
RhoA/Rho kinase
what are Rho proteins
monomeric G proteins that exchanges GDP and GTP
how is Rho activated
a exchange factor exchanges GDP on Rho to GTP
what is the role of activated Rho
activated GTP-Rho activates Rho kinase
activates GTPase GAP to degrade GTP (slow process) to return to OFF state of Rho
what complex can maintain the OFF activity of Rho
GDI (partner protein) binds to Rho-GDP and maintains its inactivity
what does RhoK (kinase) inhibit to maintain contracted state
MLCP = phosphatase
MLC20 remains in phosphorylated state longer
how does RhoK (when activated by GTP-Rho (by GEFs)) act on the contraction
Rho kinase phosphorylates :
- CPI 17 -> inhibiting activity of MLCP -> no dephosphorylation of MLC20
- MLCP -> remains in inactive form -> no dephosphorylation of MLC20
- MLC20 -> contraction
what is the CML contraction-relaxation cycle
Increase of intracellular Ca -> Ca + CaM -> Ca-CaM + MLCK -> +ATP -> MLC phosphorylation -> phosphoMLC + actine -> phospho-actomyosine -> contraction
RhoA-GDP -> GEFs -> RhoA-GTP (active) -> Rho kinase -> inhibit MLCP (directly by phodpho or indirectly by CPI17 phospho) -> phosphoMLC -> contraction
so overall what are the processes involved in excitation-contraction coupling in CML
L type CA channel/ionotropic receptors/SAC -> entry of Ca
ANGII binds to RCPG (type Gq11) -> PLC -> PIP2 -> IP3 + DAG
IP3 -> binds to RIP3 -> Ca exit of sarcoplasmic reticulum
DAG -> binds to SMOC -> Ca entry into cyoplasm
Ca binds to RYR2/3 on SR -> exit of Ca of SR
Ca activates MLCK -> phospho MLC20 -> contraction
ANGII binds to RCPG (Gq11) -> activates GEFs -> RhoA-GTP -> Rho Kinase -> inhibits MLCP -> contraction
what is passive relaxation of CML
inactivation/stopping of excitation-contraction mechanisms to make exit Ca from cytoplasm or enter SR (stockage)
what ATPase/pumps/exchangers are involved in passive relaxation
SERCA (SR Ca ATPase)
PMCAs (plasma membrane Ca ATPase)
Na/ CA exchangers
mitochondria
how does Na/Ca exchanger act
for one Ca2+ that leaves the cell 3 Na+
how does PMCA and SERCA work
Mg enters and Ca leaves (ATP) the cytoplasm -- PMCA
Mg leaves RS and Ca enters (+ATP) -- SERCA
what is active relaxation
where a signal/molecules acts on the CML to induce relaxation
What factors can lead to vasodilation in smooth muscle cells?
BNP,CNP,ANP and neuronal nitric oxide (NO).
how does A/B/CNP act on relaxation
they bind to receptors w/ enzymatic activity (guanylate cyclase)
- sGC -> cGMP -> PKG (relaxation)
What are the effects of nitric oxide (NO) on smooth muscle?
NO diffuses across membrane and induces vasodilation by increasing cGMP levels, which activates MLCP and promotes relaxation.
what is the activity of PKG
kinase that phosphorylates & inactivate contractile actors or activate relaxing factors
how does PKG act in active relaxation
when activated by cGMP -> PKG acts on :
- inhibiting IP3 mediated pathway
- increase entry of Ca into SR
- decrease intracellular Ca -> decreased activity of MLCK -> myosin head detaches from actin -> relaxation
- inhibits RhoA
What is the role of cyclic guanosine monophosphate (cGMP) in smooth muscle relaxation?
cGMP activates protein kinase G (PKG), which promotes MLCP activity and leads to relaxation.
how do beta adrenergic/prostacyclin receptors induce vasodilation by adenylate cyclase
adrenaline binds to receptors -> activate adenyl cyclase -> ATP turned into AMPc -> activates PKA -> phosphorylates MLCK -> MLC 20 not phosphorylated -> relaxation
what protein G is involved in beta adrenergic and prostacyclin receptors
protein Gs
what are the main metabolism pathways in a CML
1. gluc entry by GLUT1 -> glycolysis
2. NO synthesis
3. fatty acid synthesis
4. Pentose phosphate pathway -> nucleotides
What is the role of sympathetic nervous regulation in blood vessels?
Sympathetic activation typically leads to vasoconstriction or vasodilation through norepinephrine/noradrenaline NE and EPI epinephrine/adrenaline
What is the role of sympathetic nervous regulation in blood vessels on alpha-adrenergic receptors
vasoconstriction induced by NE/NA
What is the role of sympathetic nervous regulation in blood vessels on beta-adrenergic receptors
vasodilation induced by NE/NA
how does the neurotransmitters get to the CML through parasympathetic system
through the dendrites of the axon of postganglionic autonomic neuron
What is the primary effect of parasympathetic nervous regulation on blood vessels?
It induces vasodilation primarily through NO release from endothelial cells via the M3 receptor by Ach
in the parasympathetic nervous regulation what activates M3 endothelial cells
Acetylcholine -> M3 receptor -> PLC -> PIP2 -> IP3 -> RIP3 on SR -> release of Ca into cytoplasm -> Ca + CaM -> eNOS -> NO production and diffusion
the NO produced in the parasympathetic nervous regulation acts on CML
when produces in CE -> diffusion -> sGC -> cGMP -> activates PKG -> vasodilation
what are the main vasoconstrictors
adrenaline/NA/ANGII -> alpha adrenergic receptors -> PLC, IP3, RhoA/RhoK
What are the main vasodilators?
adrenaline/ANP/PG12 -> beta-adrenergic receptor -> AMPc
How do action potentials in smooth muscle cells differ from those in skeletal muscle?
They are typically slower and can involve various types of ionic channels.
what is passive relaxation of CML
inactivation/stopping of excitation-contraction mechanisms
what ATPase/pumps/exchangers are involved in passive relaxation