Crystalline and Amorphous Solids
0.0(0)
0.0(0)
Card Sorting
1/5
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
6 Terms
1
New cards
Crystalline solids
The solids featuring highly ordered arrangements of their particles
•Salt, diamond, sodium nitrate, ice, copper sulfate, graphite, sugar
2
New cards
Amorphous solids
The solids in which the particles are not arranged in any specific order
Upon cooling, amorphous solids turn into a brittle glass-like state
3
New cards
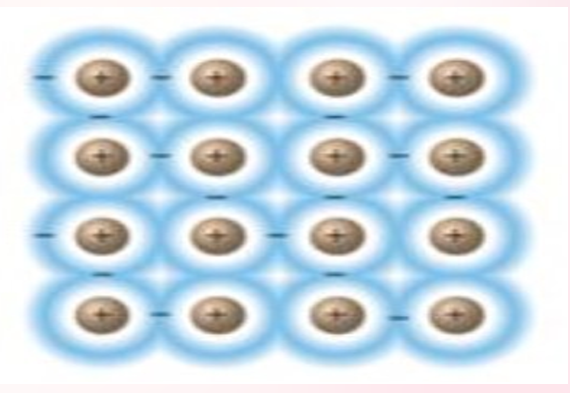
METALLIC CRYSTALS
4
New cards
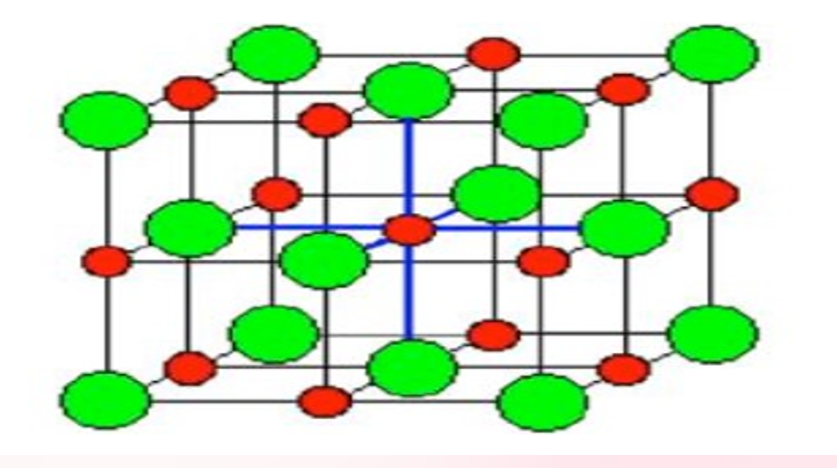
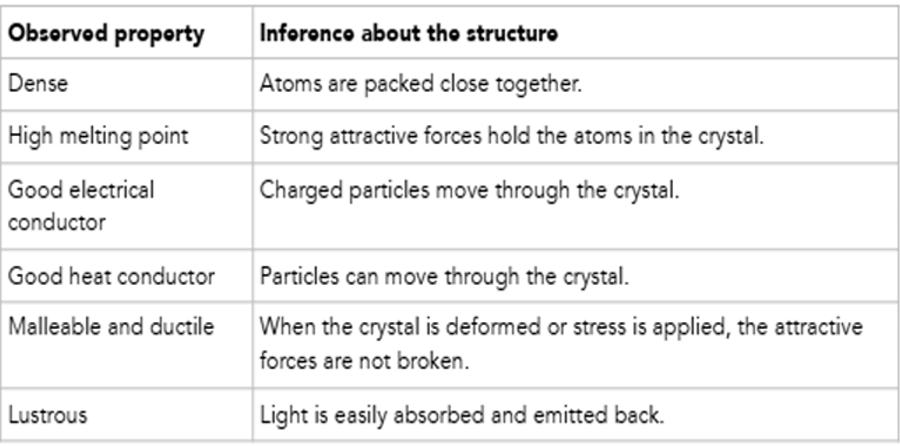
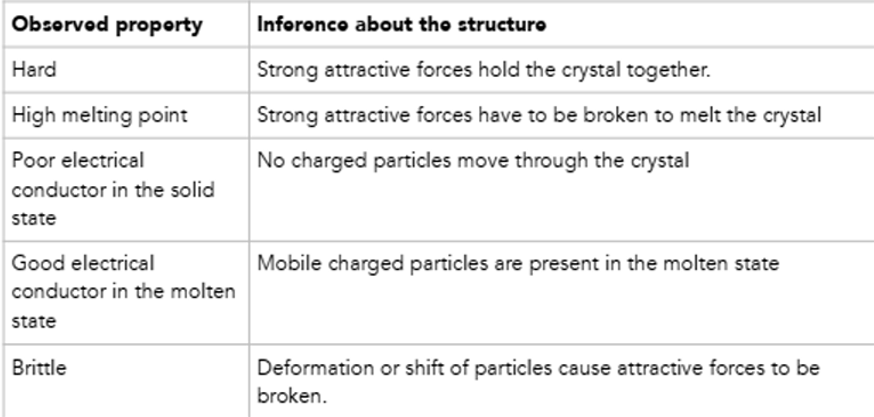
IONIC CRYSTALS
5
New cards
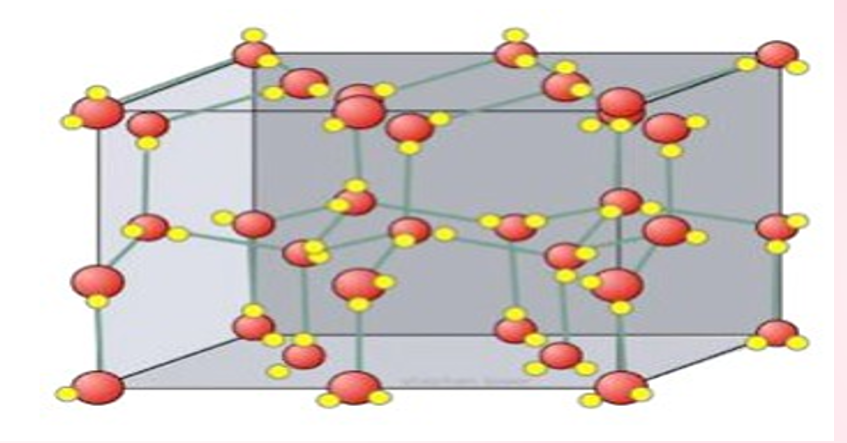
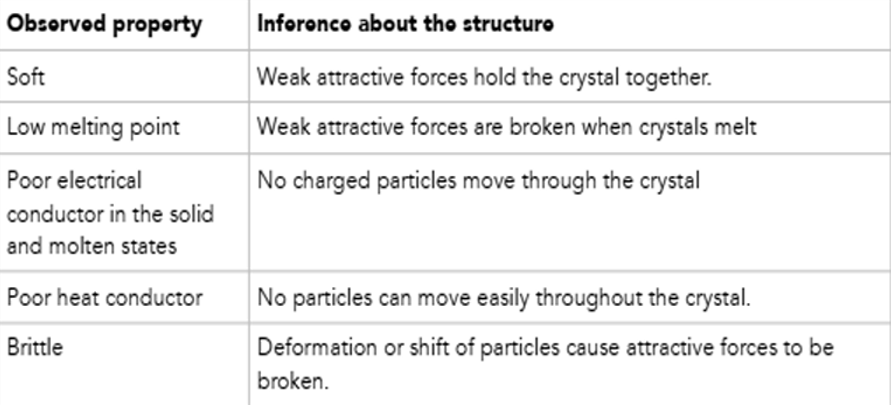
MOLECULAR CRYSTALS
6
New cards
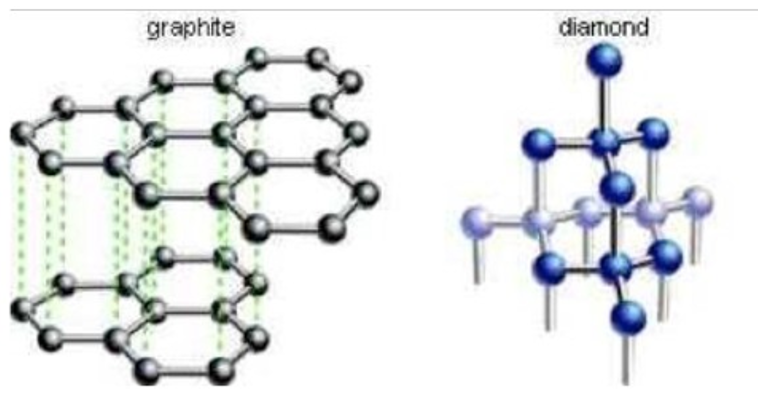
COVALENT NETWORK CRYSTALS