16. Photoreceptor Physiology
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
51 Terms
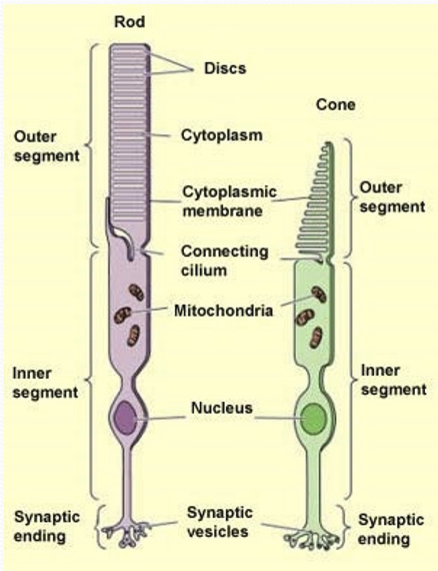
Where is protein production and oxidative metabolism restricted to?
Inner segment
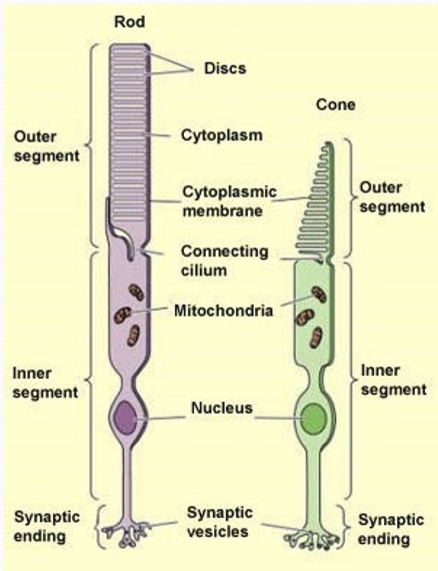
Where is the biochemical cascade of phototransduction restricted to?
The outer segment
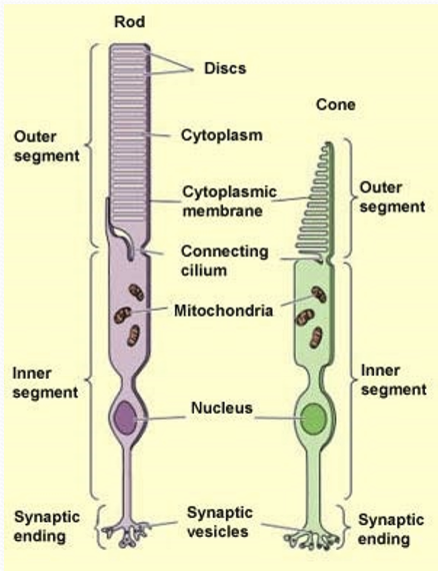
What connects the outer and inner segment?
A connecting cilium, containing microtubules.
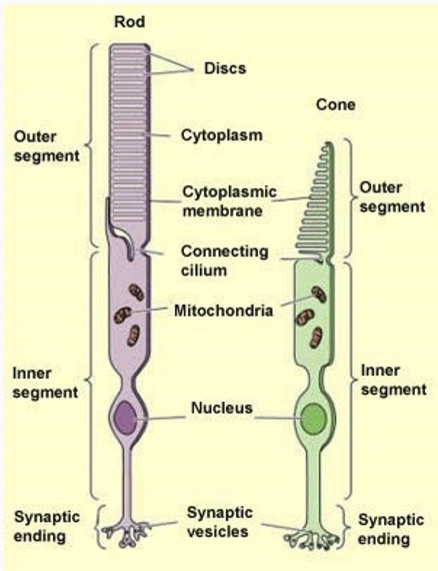
What is the cilium used for?
Transport of cellular components and ion flow.
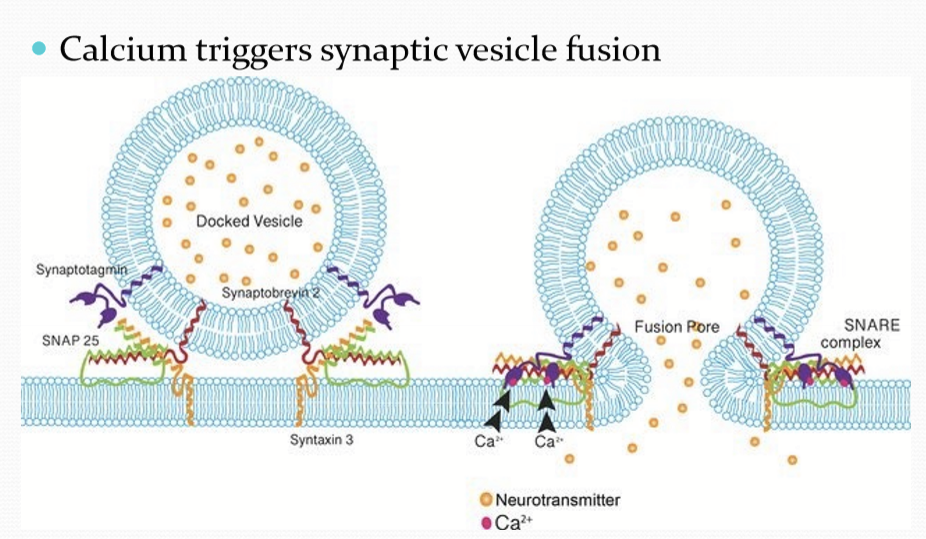
What does intracellular calcium induce?
Synaptic vesicle fusion with membrane.
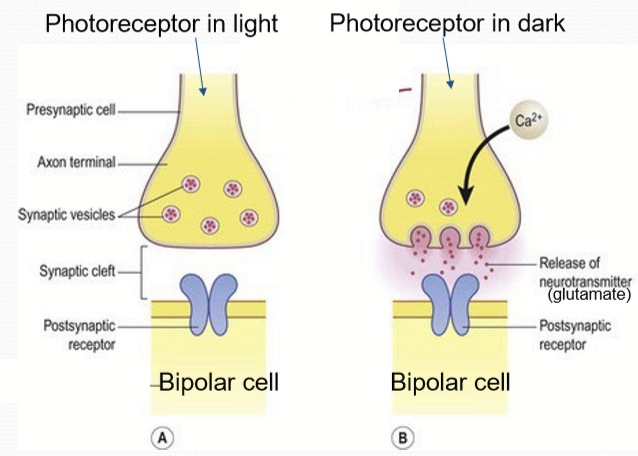
What does the phototransduction pathway ultimately regulate?
Intracellular calcium ion levels. In light, extremely reduced calcium= reduced synaptic fusion. In dark, higher calcium ions= more synaptic fusion.
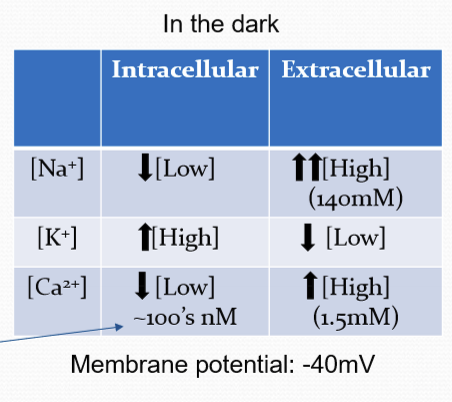
What is the membrane potential of a non-illuminated rod?
Partially depolarized. Resting membrane potential of approx. -40 mV.
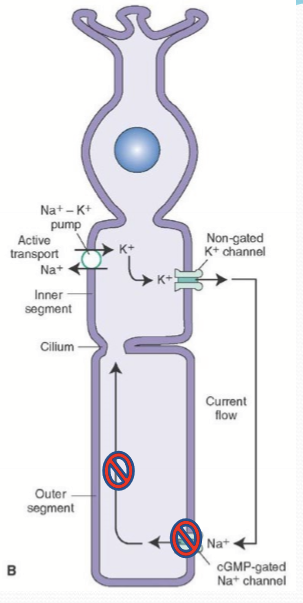
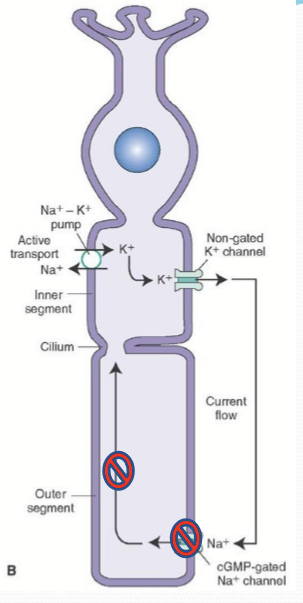
Where is NKA found on a photoreceptor?
Inner segment.
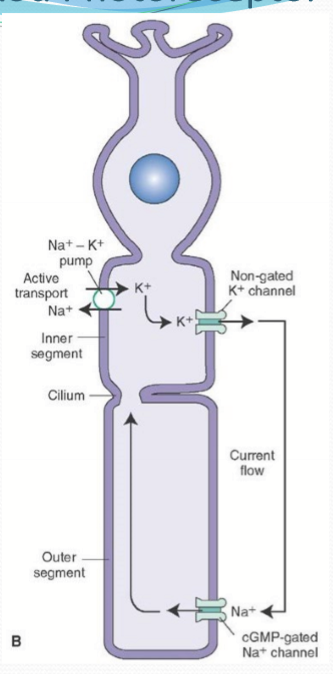
What does the potassium channel of the inner segment do?
Allows the flow of K ions back out of the photoreceptor cell.
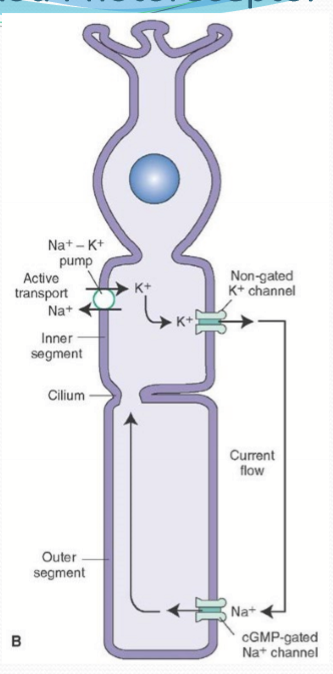
What does the cyclic nucleotide-gated channel (CNG) do?
It opens when bound to cGMP intracellularly to allow Na, K, and Ca pass into the cell. It is responsible for partial depolarizaion.
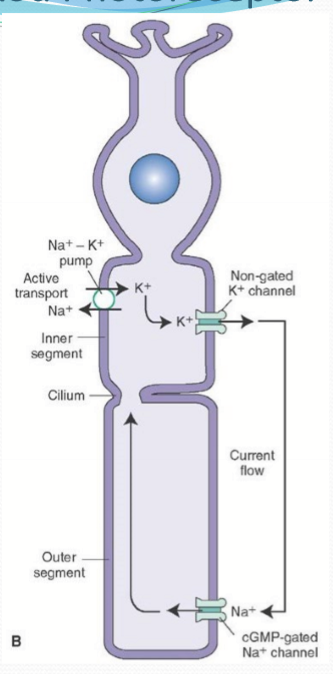
What is the dark current?
The net flux of cations. With K going out of the inner segment and Na/Ca entering in the outer segment through CNG.
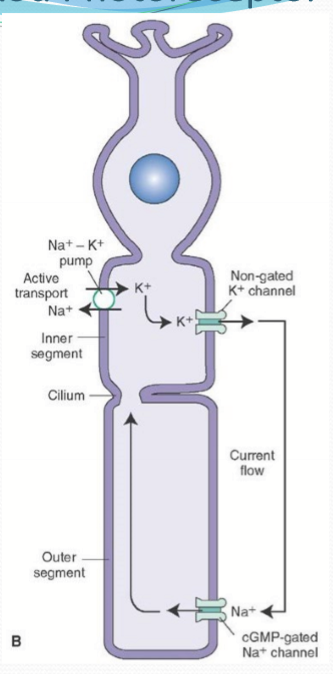
What ks NCKX?
An exchanger in the outer segment that moves 4 Na into the cell and 1 K & 1 Ca out of the cell.
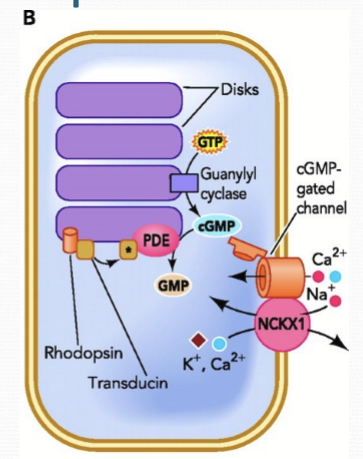
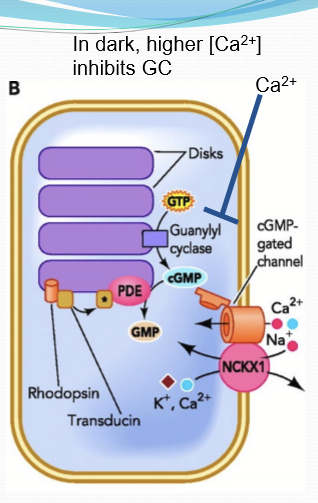
How is cGMP regulated?
Opposing enzymatic activities: Guanylate cyclase (GC) synthesizes cGMP to open CNG; phosphodiesterase (PDE) degrades cGMP to GMP, closing CNG.
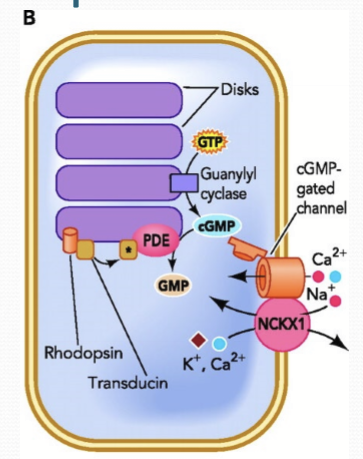
Where is GC/PDE localized to?
The segment disc membranes of cones and rods.
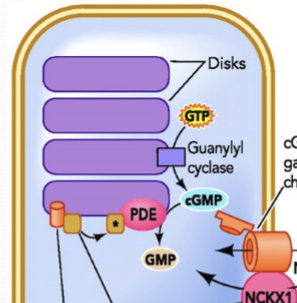
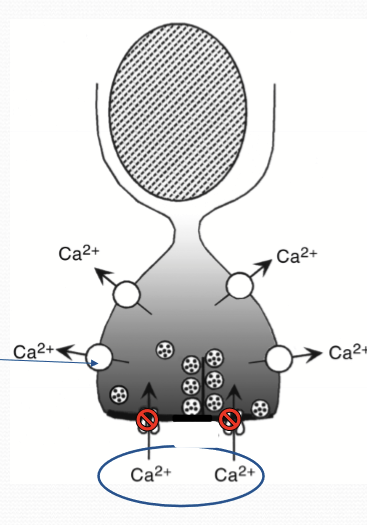
What happens to calcium in the photoreceptors in the dark?
Photoreceptors are constantly firing and releasing glutamate into synapes because Ca channels remain open in the dark.
How is Ca regulated?
Outer segment: CNG & NCKX
Synaptic region/inner segment: Synaptic region, voltage-gated calcium channels, PMCA
How does the Voltage-gated Ca channels work?
Depolarized state (dark) allows Ca to flow into the cell. Hyperpolarized cell (illuminated) closes the channel.
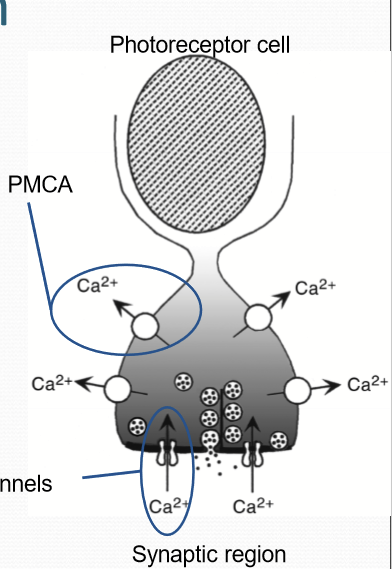
How does PMCA (plasma membrane calcium ATPase) work?
Functions independently of illumination, constantly transports Ca outside of cells. Is ATP dependent instead.
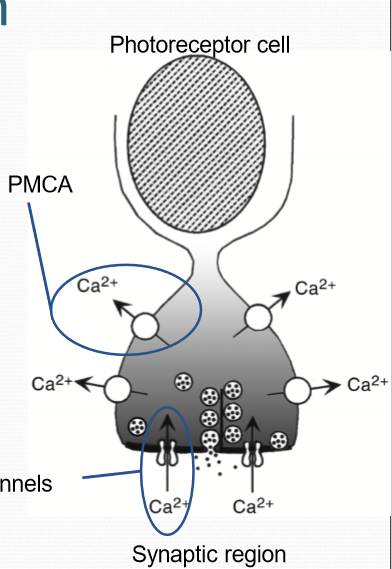
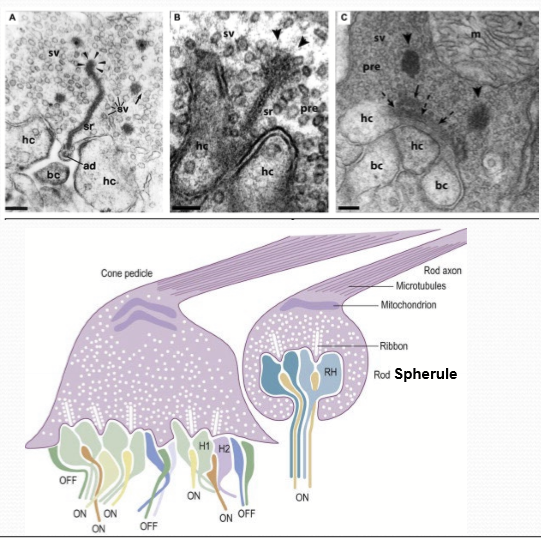
What are synaptic ribbon?
Structure designed to mediate high volume, tonic release of synaptic vesicles. Tethered to numerous synaptic vesicles.
What is rhodopsin composed of?
Retinal + Opsin coupled with a GPCR
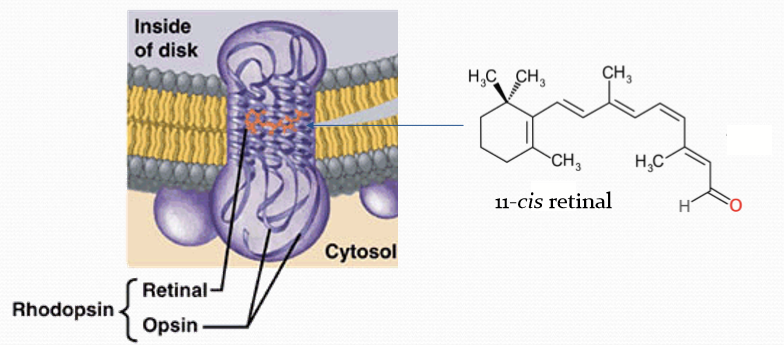
Where is rhodopsin located?
In the disk membrane.
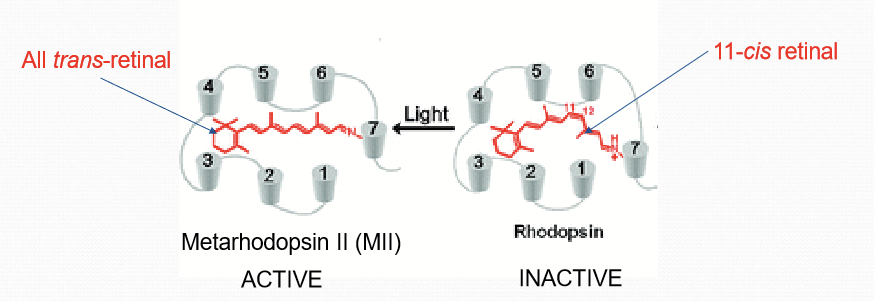
When is rhodopsin inactive?
When bound to 11-cis retinal.
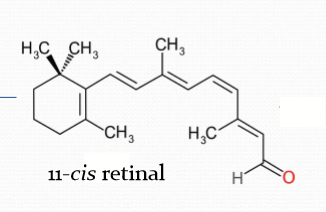
How does rhodopsin respond to light?
When it absorbs a photon of light, it becomes 11-cis retinal to all trans-retinal. The conformation change converts rhodopsin to be converted to Metarhodopsin II.
What happens after rhodopsin converts to Metarhodopsin II?
It activates G-protein Transducin, converting it from GDP-bound to GTP-bound form (active).
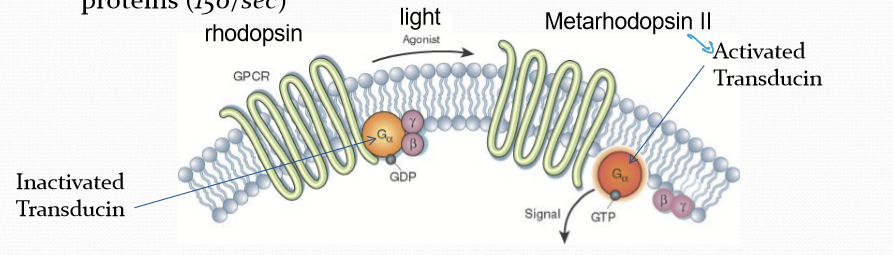
How many Metarhodopsin II molecules can catalytically activate many G proteins?
150/sec
What does transducin-GTP do after its been activated?
It increases its affinity for phosphodiesterase 6 (PDE6). Binding to PDE6 increases its catalytic activity 1000 fold and allows it to covert all cGMP to GMP in 0.1 seconds.
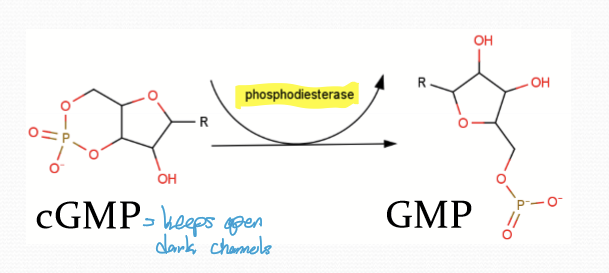
What happens after transducin-CTP binds to PDE6?
cGMP conc decreases and CNG channel closes.
How sensitive id CNG?
Very, a small reduction in cGMP has a large effect on channel closure due to constant dissociation of cGMP from CNG.
When the CNG channel closes, what happens to the cell?
Reduced flow of Na+ ions into the cell, making it hyperpolarized to -70mV (from -40). This reduces the dark current.
What does hyperpolarization cause?
Voltage gated channels to close and gluatmate release is reduced
Calcium levels also drop to very low levels
Ca is still being pumped out via PMCA and NCKX.
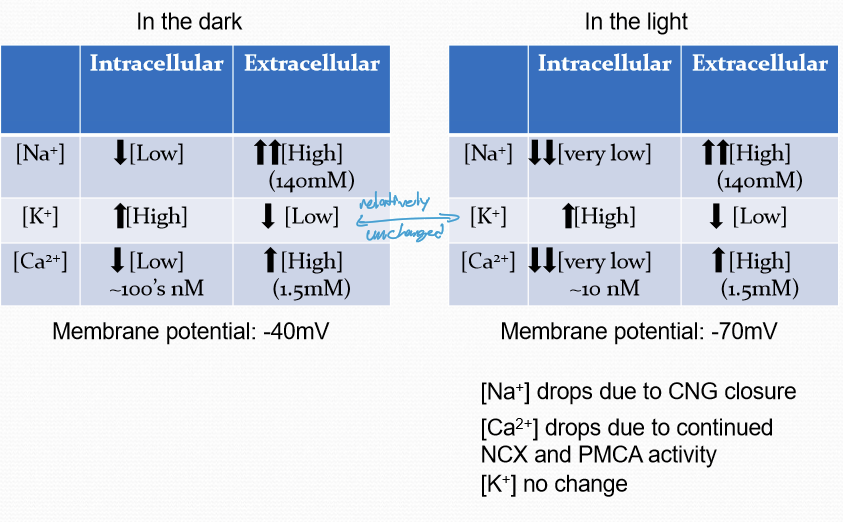
What are the differences between Na, K, and Ca when the cell is in the dark vs light?
Na drops due to CNG closure
Ca drops due to continued PMCA and NCKx activity
K has no change
What happens in photoreceptors when they are stimulated by photons?
The response can be both additive and regional, depending on where and how many photons are absorbed.
How can a single photon affect a photoreceptor?
A single photon can efficiently cause local closure of ion channels in the plasma membrane.
What happens to CNG during light activation?
CNG can close in multiple regions based on photon absorption sites.
How do multiple photon absorption events affect glutamate release?
They have an additive effect that further slows down glutamate release from the photoreceptor.
What is saturation in rods and when does it occur?
Saturation occurs when bright light causes enough photons to be absorbed that all ion channels close.
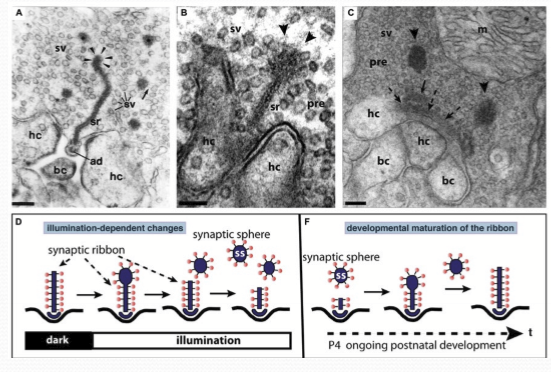
What happens to the synaptic ribbons when light is present?
Decreases in size. This is due to the snaptic vesicles dissociateing from it.
What is required to reset the system to allow another light stimulus?
Inactivation of MII
cGMP restoration
Transducin and PDE6 inactivation
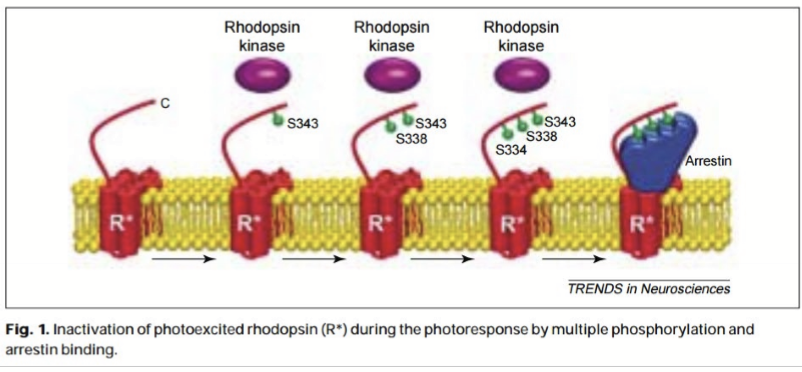
How is MII deactivated?
Rhodopsin kinase is phosphorylated (RK1).
Arrestin bins and prevents efficient association with transducin, inactivating metarhodopsin/MII.
Schiff;s base hydrolysis all trans retinal from MII; REleases of all-trans retinal from MII and removes arrestin from rhodopsin. Opsin can re-associate with a new 11-cis retinal.
How is cGMP restored?
Guanylyl cyclase activity increases with lowering of cytoplasmic Ca, increasing cGMP levels, causing CNG channels to open again.
What does high conc calcium do to guanylyl cyclase?
It inhibits its activity through calcium-binding proteins.
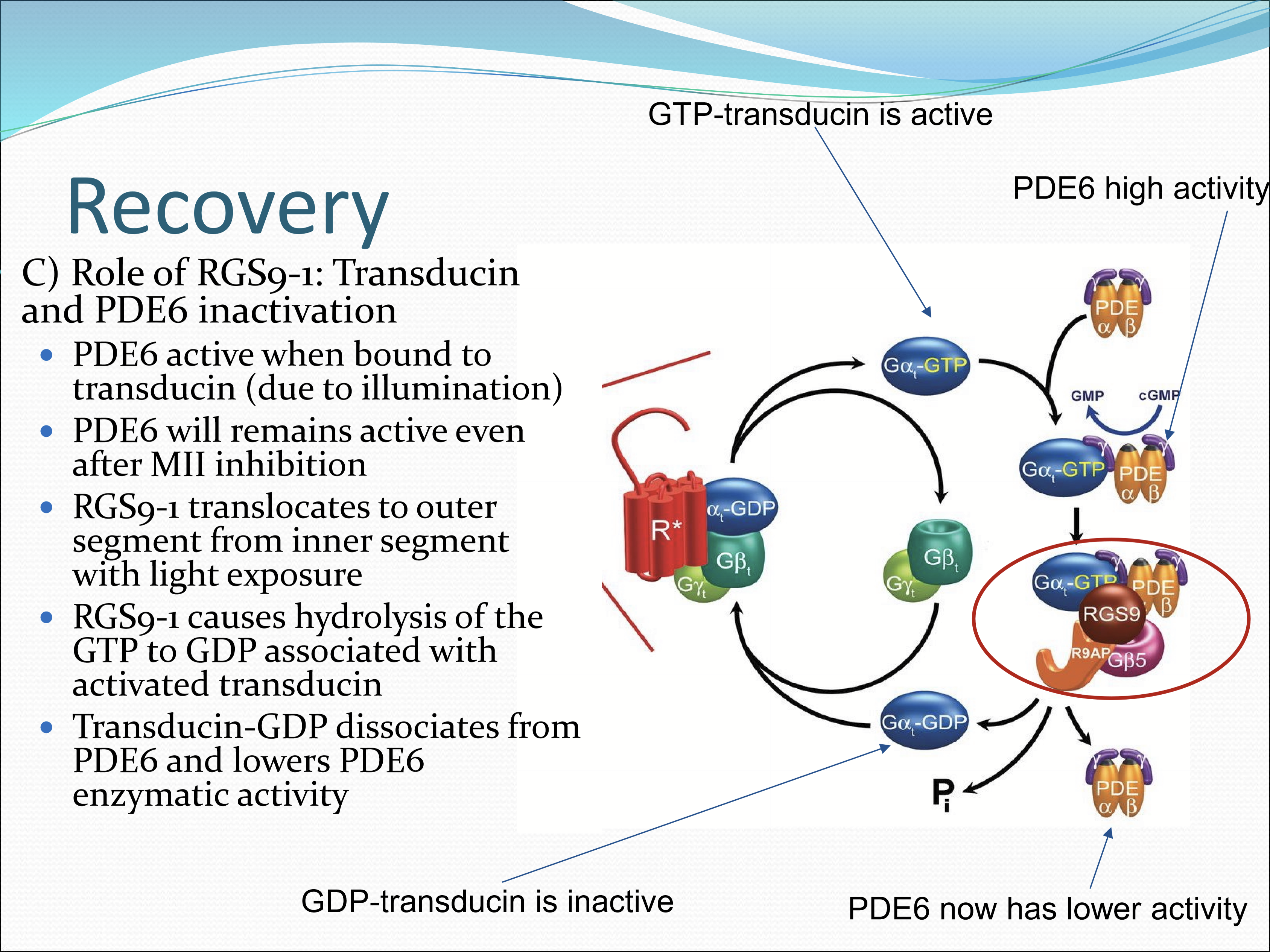
What is the role of RGS9-1?
Inactivates transducin and PDE6. It translates to the outer segment from the inner segment with light exposure. RGS9-1 cuases hydrolysis of the GTP to GDP associated with activated transducin. Transducin-GDP dissociates from PDE6 and lowers PDE6 enzymatic activity.
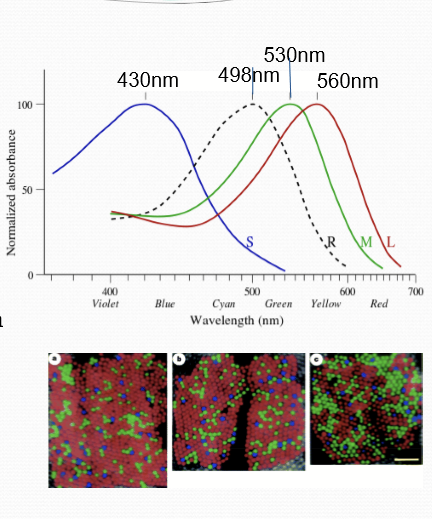
What is the difference between rods and cones?
Distinct opsin proteins
Rod opsins called rhodopsin, cones are S-opsin-blue; M-opsin-green; L-opsin-red
Different interactions with 11-cis retinal and polarity of amino acids next to its binding pocket
Location: only cones in fovea. Cone photoreceptor density decreases as the distance from the fovea increases.
What causes red-green color vision defects?
Alterations in L-opsin and/or M-opsin genes on the X chromosome lead to absence of L or M cones or the production of abnormal opsin pigments in the cones that affect color vision.
What is the loss/limited L-cone (red) called?
Protan
What is the loss/limited M-cone (green) called?
Deutan
What can cause blue-yellow color vision defects?
S-opsin gene= tritan
Premature destruction of S cones or the production of defective S cones
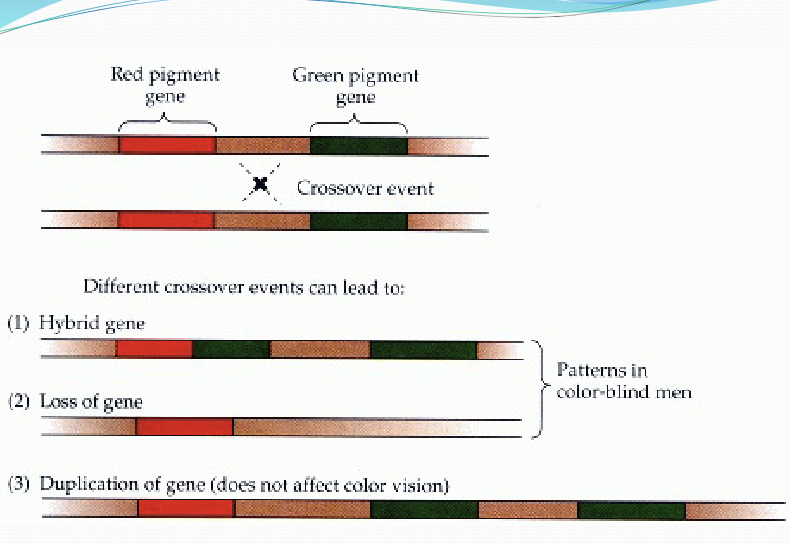
What are the types of monochromacy?
cone monochromacy: 2/3 cone photopigment genes don’t work, can be red/green/blue monochromacy
rod monochromacy (achromatopsia): functional disruption of all cone types; mutations in cone-specific phototransuction protein encoding genes: Transducin, CNG, PDE.
What are rods more sensitive than cones to light?
Fewer photons necessary to elicit similar response in rods vs cones
Cone response must rise about the noise, as spontaneous isomerization rates of cone opsins are greater than rhodopsin
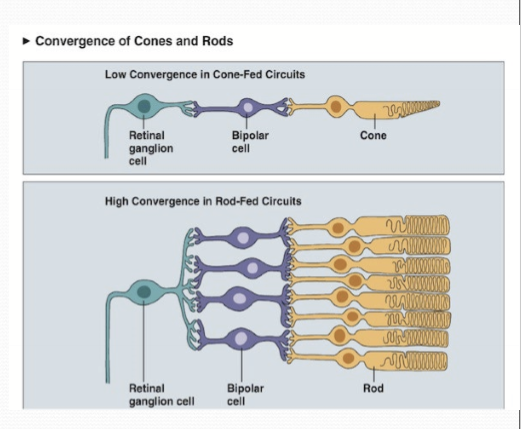
Convergence of photoreceptors to a single retinal ganglion cell is greater in rods than cones. Any photon within several rods will trigger an action potential in a RGC.
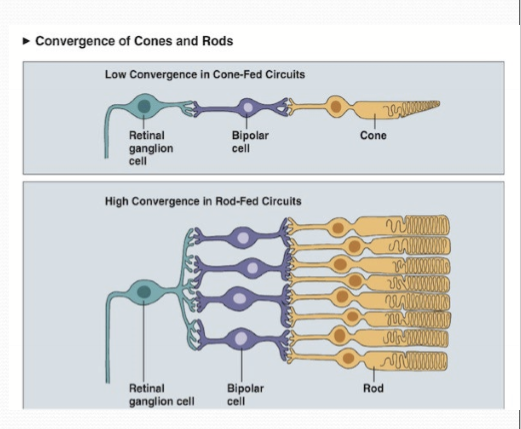
What has a higher spatial and temporal acuity?
Cones, due to lower convergence of cones on RGCs. Temporal acuity due to speed of phototransduction; faster recovery allows cones to respond with a greater range.
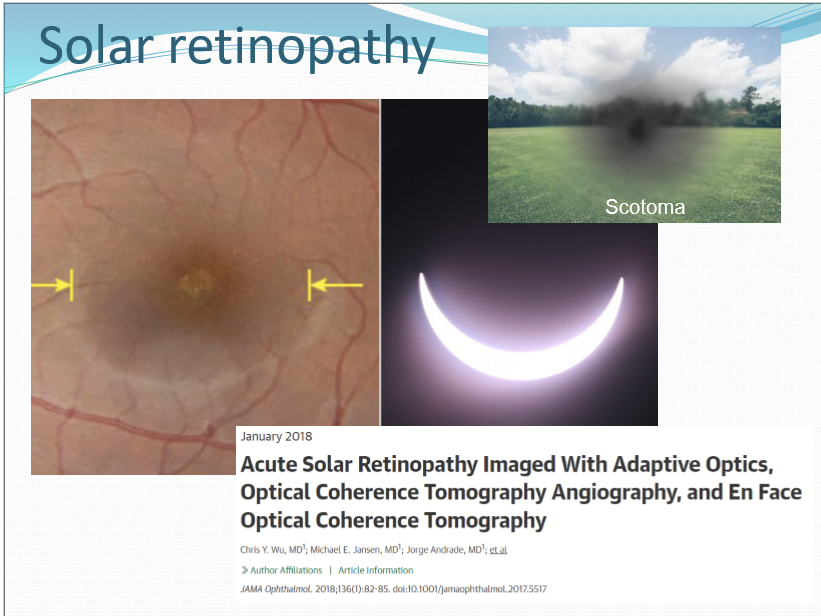
What is solar reetinopathy?
oof