🫁 Alveolar Gas Exchange 2
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
define gas transport in the cardiovascular system?
Gas transport = movement of respiratory gases within cardiovascular system.
Involves processes and mechanisms related to delivery of O₂ and CO₂ between pulmonary and tissue compartments.
how do oxidative metabolism and metabolic rate affect O₂ and CO₂ movement?
Oxidative metabolism converts fuel to energy and is required for cellular function.
Transport rates operate on metabolic supply and demand.
↑ metabolism → ↑ O₂ consumption → ↑ O₂ delivery.
↑ metabolism → ↑ CO₂ production → ↑ CO₂ removal.
What causes gas transport imbalances and what are the consequences?
Tissues must match O₂ and CO₂ used/produced with what enters/exits lungs.
Imbalance results from lung disease or exercise.
Exercise raises metabolism; if lungs cannot increase O₂ intake or CO₂ removal → imbalance.
↓ blood [O₂] = hypoxemia
↑ blood [CO₂] = hypercapnia (acidosis)
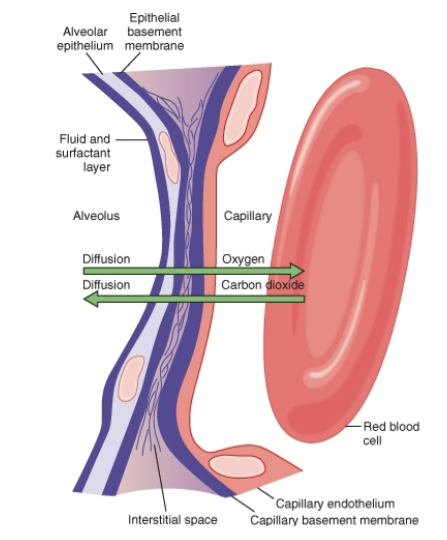
What layers make up the respiratory membrane?
Layer of fluid (mostly surfactant)
Alveolar epithelium
Epithelial basement membrane
Thin interstitial space between alveolus & capillary
Capillary basement membrane
Capillary endothelial membrane
Additional slide details:
Membrane thickness 0.2–0.6 μm
Surface area ~70 m² in average male
60–100 mL blood spread over 25 × 30 ft floor
What factors limit diffusion capacity?
Diffusing properties (Fick’s law) of membrane and gas
Fick’s equation in the lung
Blood carrying properties (e.g., hemoglobin)
What does Fick’s law state on the slide?
Rate of diffusion depends on:
Partial pressure difference
Diffusion constant (includes MW + solubility)
Surface area
Membrane thickness
Formula shown:
Diffusion (D_gas) = (ΔP × area × solubility) / (thickness × MW)
Occurs via simple diffusion between:
Alveoli (air) ↔ blood
Arterial blood ↔ tissue (mitochondria)
JW HY:
super crazy high mountains, like Denali and McKinley, where people go and actually start using O2, it's because at that altitude, if I'm at a partial pressure of 500 times .21 for my O2,
my delta P for gas exchange is getting lower and lower and lower.

What membrane properties does the slide emphasize? LY
Membrane properties vary during respiratory cycle.
Large surface area ~80 m².
Very thin ~0.3–0.6 μm.
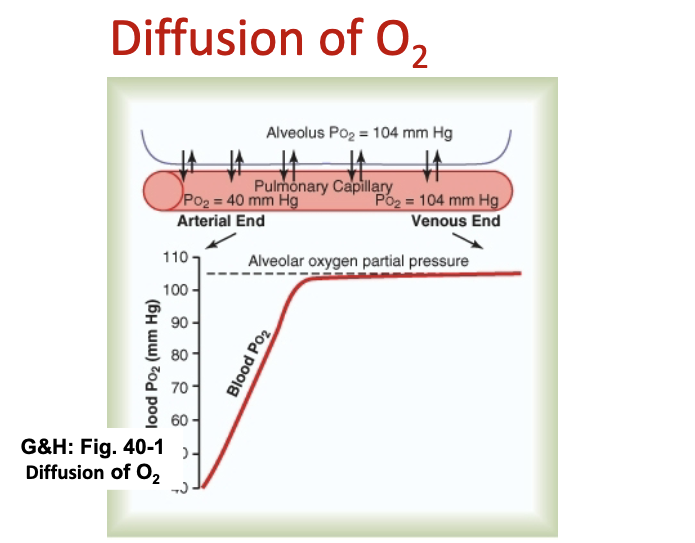
What does the slide show about O₂ equilibration in the capillary?
Steep partial pressure gradient varies during respiratory cycle.
O₂ reaches equilibrium within 0.25 sec, about 1/3 through the capillary bed.
What does the O₂ diffusion slide depict?
Graph demonstrates O₂ diffusion from alveoli to blood
O2 reaches equilibrium within 0.25sec. or approximately 1/3 the distance through the capillary bed.
JW HY:
So what you have to remember is this is a pulmonary capillary.
pulmonary artery Is deoxygenated blood coming from the right heart.
The pulmonary vein is going into the Left atria to pump this oxygen-rich blood into the systemic circulation.
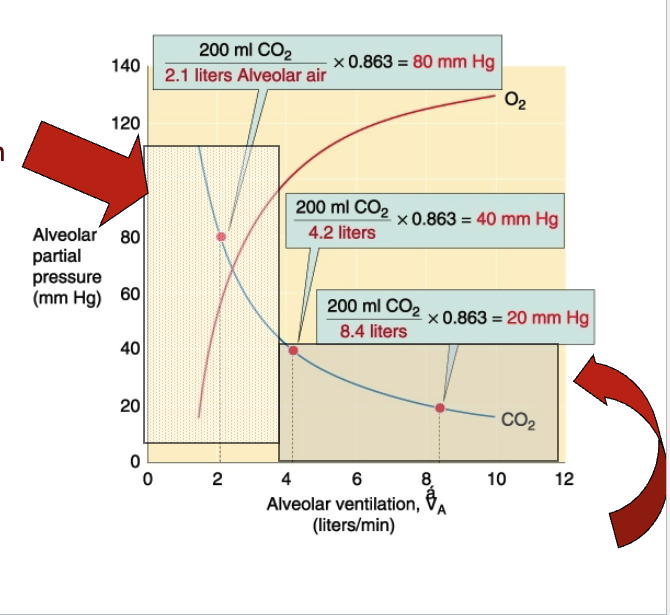
What does the slide say about CO₂ diffusion at the respiratory membrane?
CO₂ reaches equilibrium within 0.25 sec (≈1/3 capillary distance).
Partial pressure differences for CO₂ are smaller, but CO₂ diffuses ~20× more rapidly due to higher solubility.
Therefore, CO₂ reaches equilibrium roughly same time as O₂.
What are the slide’s definitions of hyperventilation and hypoventilation?
Hyperventilation: ↑ V_A with constant VCO₂ → hypocapnia → can lead to alkalemia (↓ H⁺).
Hypoventilation: ↓ V_A with constant VCO₂ → hypercapnia → can lead to acidemia (↑ H⁺).
What reactions occur at tissues and at gas exchange sites?
At tissues:
substrate + O₂ → CO₂ + H₂O + H⁺ + heat + ATP
At gas exchange sites:
CO₂ + H₂O ↔ H₂CO₃ ↔ H⁺ + HCO₃⁻
What does the slide state about O₂ dissolved in plasma?
Henry’s law: dissolved gas proportional to partial pressure.
O₂ not very soluble → only 0.3 vol% dissolved in plasma.
Calculation on slide:
VO₂ = 5000 mL/min × 0.3 mL O₂ per 100 mL = 15 mL O₂/minAvg 70 kg human consumes 250 mL O₂/min at rest.
If O₂ only in plasma → resting metabolism would need to drop 17×.
Leads to question: How do we increase O₂ carrying capacity?
JW HY: So the way that we are going to increase our carrying capacity is through hemoglobin.
What hemoglobin properties are listed?
Stored in RBC: 15 g/100 mL blood
1.35 mL O₂/g Hb
4 subunits: 2α + 2β chains
Each subunit has heme with iron atom
Cooperative O₂ binding
Large O₂ binding affinity → rapid binding
O₂ binding reversible
Binding is PO₂-dependent
Iron must be Fe²⁺ for O₂ reversible binding
Methemoglobin (Fe³⁺) does not bind O₂
What differences between Mb and Hb are listed?
Both:
Interact with O₂
Increase O₂ solubility
Contain heme
Myoglobin:
Found in striated/skeletal muscle
Monomeric → one O₂ site
Stores O₂ in cytoplasm
Delivers O₂ to mitochondria on demand
Hemoglobin:
In RBCs only
Heterotetramer
4 O₂ binding sites
Transports O₂ lung → tissues
What does the slide illustrate about the curves?
Graph comparing Mb vs Hb saturation curves (no text beyond figure).
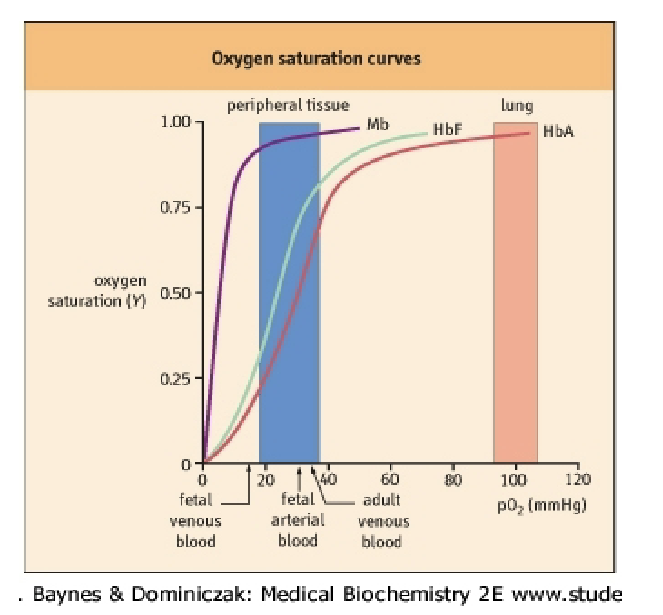
What is the relationship between Hb saturation and O₂ capacity?
Hb responsible for O₂ capacity.
O₂ capacity ≈ 1.35 mL O₂/g Hb.
Max O₂ bound = 1.35 mL × 15 g/dL = ~20.3 mL O₂/dL blood.
% saturation formula:
%S O₂ = (O₂ bound to Hb / Hb O₂ capacity) × 100.Curve is sigmoidal due to cooperativity.
JW HY:
100% saturation is 100% saturation, so what are you gaining from going to this O2 bar?
Right? You're increasing the partial pressure of O2, but if you're 100% saturated on your hemoglobin.
Anemia = change in hemoglobin conc. = cap. to carry O2 reduced
What does the oxygen–hemoglobin dissociation curve show?
The curve is sigmoidal, reflecting cooperative binding of O₂ to hemoglobin.
Shows loading zone (lungs) and unloading zone (tissues).
Demonstrates how changes in PO₂ affect hemoglobin saturation and O₂ delivery.
JW HY:
I want you to notice here, at a partial pressure of 40, We are still…75% saturated.
So what this means is that if we have normal alveolar ventilation of about 5.2 liters per minute, and we have normal cardiac output of about 5 liters per minute.
Always have some in reserve ***
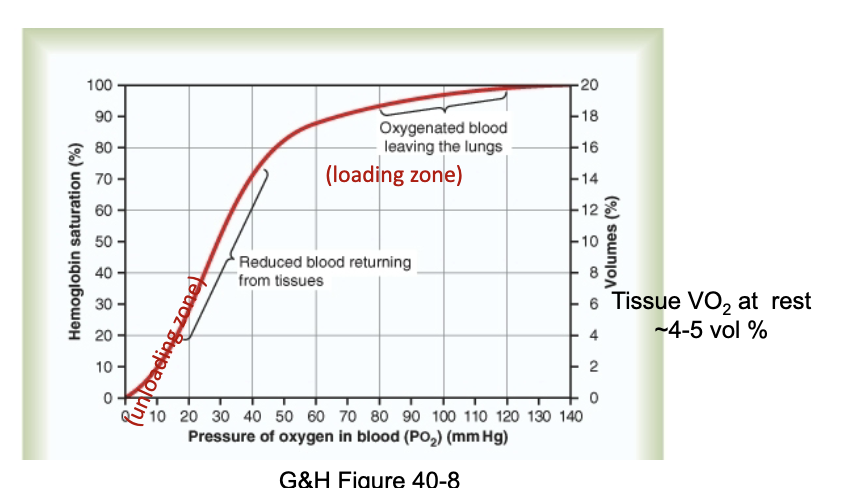
What does mixed venous PO₂ represent?
It reflects the average metabolic activity of all tissues.
The typical mixed venous PO₂ is ~40 mmHg (from slide).
What mechanisms alter O₂ transport?
Blood flow (HR × SV)
a–v O₂ difference
O₂ capacity (hemoglobin content)
Anemia (↓ Hb)
Polycythemia (↑ Hb)
Curve shifting (Bohr effect)
Effects of:
CO₂
pH / H⁺
Temperature
2,3-DPG
Any combination of these factors.
What is P50, and how do allosteric effectors change it?
P50 = PO₂ at which 50% of Hb is saturated.
Positive allosteric effector (O₂):
↓ P50
Left shift
↑ affinity
Negative allosteric effectors:
H⁺ (pH)
CO₂
2,3-BPG
Temperature
→ All increase P50, decrease affinity, and shift curve right.
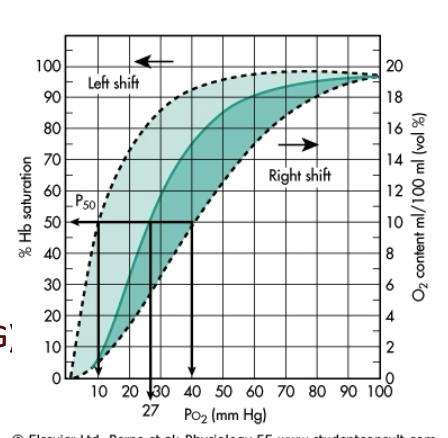
What does the figure on this slide represent?
Diagram of the factors that shift the oxyhemoglobin dissociation curve, showing left vs right shifts.
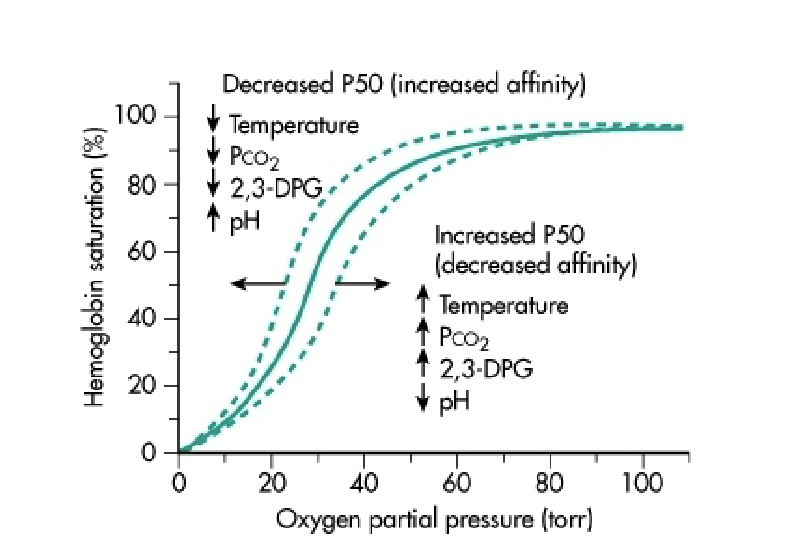
What is the Bohr effect, and how does pH influence Hb–O₂ binding?
Decreased pH (↑ H⁺) → Right shift of curve.
Increases P50 → Decreases affinity.
At lungs: high PO₂ → O₂ binding → H⁺ released.
At tissues: increased H⁺ and lower pH → Hb (oxyHb) binds H⁺ → promotes O₂ release for metabolism.
How does CO₂ affect hemoglobin’s affinity for oxygen?
↑ PCO₂ decreases Hb–O₂ affinity → Right shift.
CO₂ forms carbamino-Hb:
Hb-NH₂ + CO₂ ↔ Hb-NH-COO⁻ + H⁺CO₂ also forms HCO₃⁻ via carbonic anhydrase.
In lungs: ↓ PCO₂ → reversal (carb. anhydrase rxn)→ ↑ O₂ affinity.
What effects of 2,3-BPG are listed?
Negative allosteric effector → ↑ P50 → ↓ affinity.
Present in concentration similar to Hb (~4.1 mmol/L).
Without 2,3-BPG, Hb behaves like myoglobin.
One 2,3-BPG binds between β1 and β2 → stabilizes T-state.
High PO₂ collapses pocket → BPG released.
Chronic hypoxia → ↑ 2,3-BPG → more O₂ unloading.
JW HY:
When 2,3-DPG is present, we either have DPG released or in its place.
When we have less and less O2, we get more 2,3-DPG.
How does 2,3-DPG affect the O₂–Hb curve under normal and hypoxic conditions?
Normal plasma DPG keeps curve right-shifted (↓ affinity).
Hypoxia increases 2,3-DPG → further right shift → ↑ O₂ unloading to tissues.
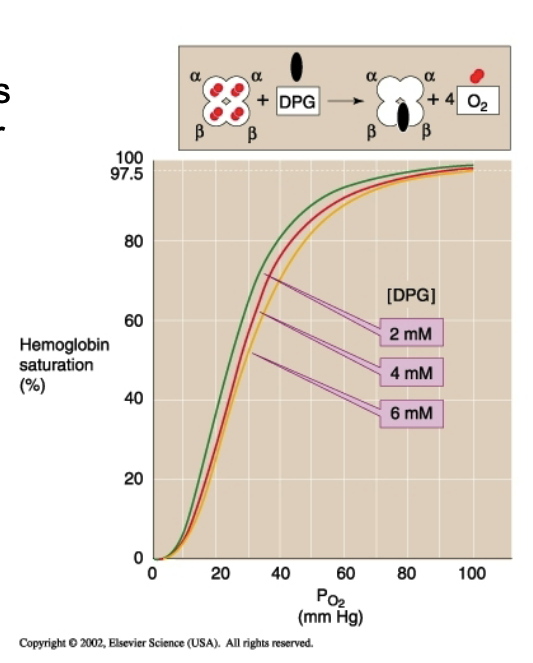
How does 2,3-DPG reduce hemoglobin’s affinity for oxygen?
It binds to and stabilizes deoxyhemoglobin (T-state), lowering O₂ affinity.
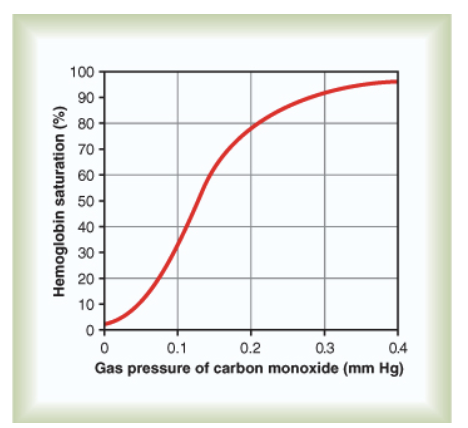
Why is CO toxic?
CO forms carbonmonoxyhemoglobin (Hb-CO), which does not take up O₂.
CO binds at same site as O₂.
CO affinity = 250× that of O₂.
Hb-CO releases CO slowly → functionally non-reversible.
Hb-CO saturation curve resembles normal but needs very low PCO; 0.6 mmHg = lethal.
JW HY:
Look at this scale. 0.4 millimeters of mercury. 0.4! So, it takes very little carbon monoxide, the partial pressure of carbon monoxide can be so small, and it will start to bind up hemoglobin, irreversible.
What physiological consequences and symptoms occur during CO poisoning?
Symptoms: headache, nausea, general hypoxia symptoms.
O₂ content low but PO₂ normal → chemoreceptors not stimulated.
Normal CO₂ diffusion preserved.
Blood appears bright red (no cyanosis).
70–80% Hb-CO → death.
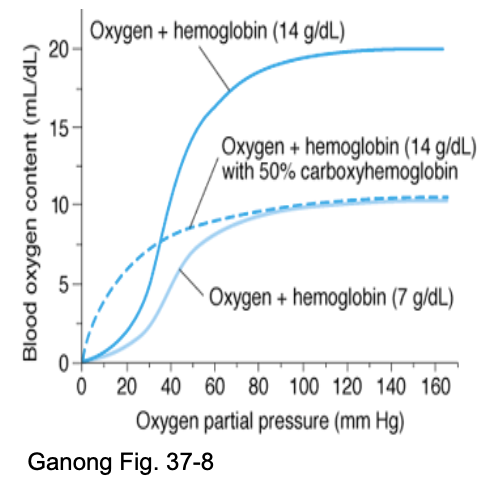
How does anemia differ from CO poisoning, based on the slide?
Anemia not severe at rest unless Hb severely decreased; worse with exercise.
RBCs ↑ 2,3-BPG → easier O₂ release.
With CO present:
Less O₂ delivered
CO binding prevents O₂ release
Curve shifts left → ↓ unloading at tissues.
JW HY:
All hemoglobin can bind oxygen at a partial pressure of 100, however, if we look at what's happening at the tissues at a partial pressure of 40, what you can see is tissue oxygen content is down, which is good. That means I'm unloading at the tissues
This person that has carbon monoxide poisoning is not able to let go of O2 level of the tissues, and this person with anemia, their hemoglobin is still functioning normally with that sigmoidal cooperative binding and being able to unload.
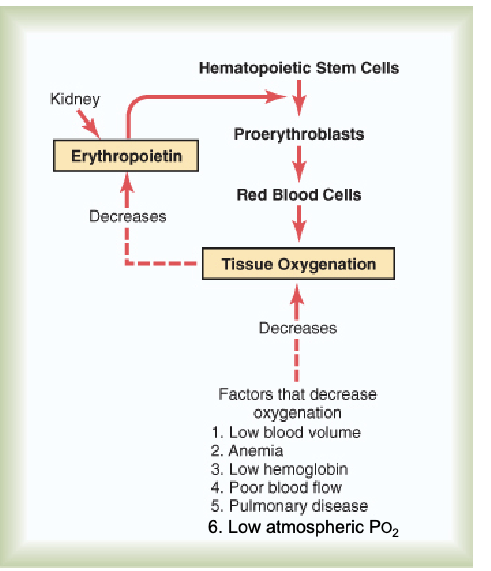
How is hemoglobin production regulated?
Local tissue hypoxia sensed by kidney triggers release of EPO.
EPO → bone marrow → stimulates hematopoietic stem cells.
EPO is a peptide hormone acting via tyrosine kinase receptors.
JW HY: Triggered by Poor blood flow, pulmonary diseases, because, you know, now you're not having good diffusion at the place where you're supposed to be having gas exchange, and low atmospheric O2.
***That low atmospheric PO₂ is a stimulus for EPO production (contextual hypoxia trigger).
What three forms of CO₂ transport are listed?
Gaseous/dissolved CO₂ in plasma
Carbamino compounds: Hb-NH-COO⁻ + CO₂
Bicarbonate (HCO₃⁻):
CO₂ + H₂O → H₂CO₃ → H⁺ + HCO₃⁻
What two mechanisms describe how O₂ binding promotes CO₂ release? Haldane effect
Acidic Hb has less affinity for CO₂ when O₂ binds.
O₂ binding releases H⁺ → drives:
H⁺ + HCO₃⁻ → H₂CO₃ → CO₂ + H₂O
→ CO₂ released from blood.
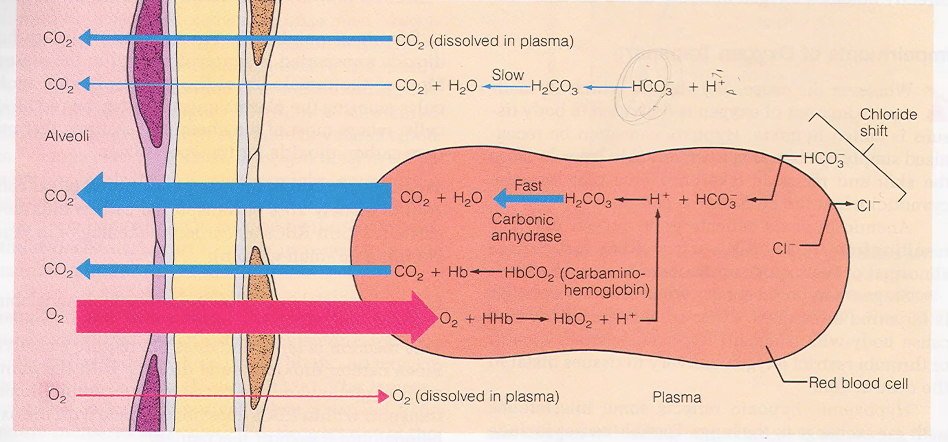
Flashcards 37–46 (Diagrams: Bicarbonate Formation, Gradients, Chloride Shift)
CO₂ production at tissues
CO₂ → H₂CO₃ → HCO₃⁻ (slow in plasma, fast in RBC via carbonic anhydrase)
HCO₃⁻ leaves RBC → electrical gradient
Chloride shift (Cl⁻ enters RBC)
Water movement into RBC (osmotic gradient)
Formation of carbamino-Hb
H⁺ binding to reduced Hb
All steps reverse in the lungs
JW HY:
So there's a chloride shift happening in the red blood cell. All of this is trying to, at the level of the tissue.
Release O2, pick up CO2, and keep this cell in its proper equilibrium.
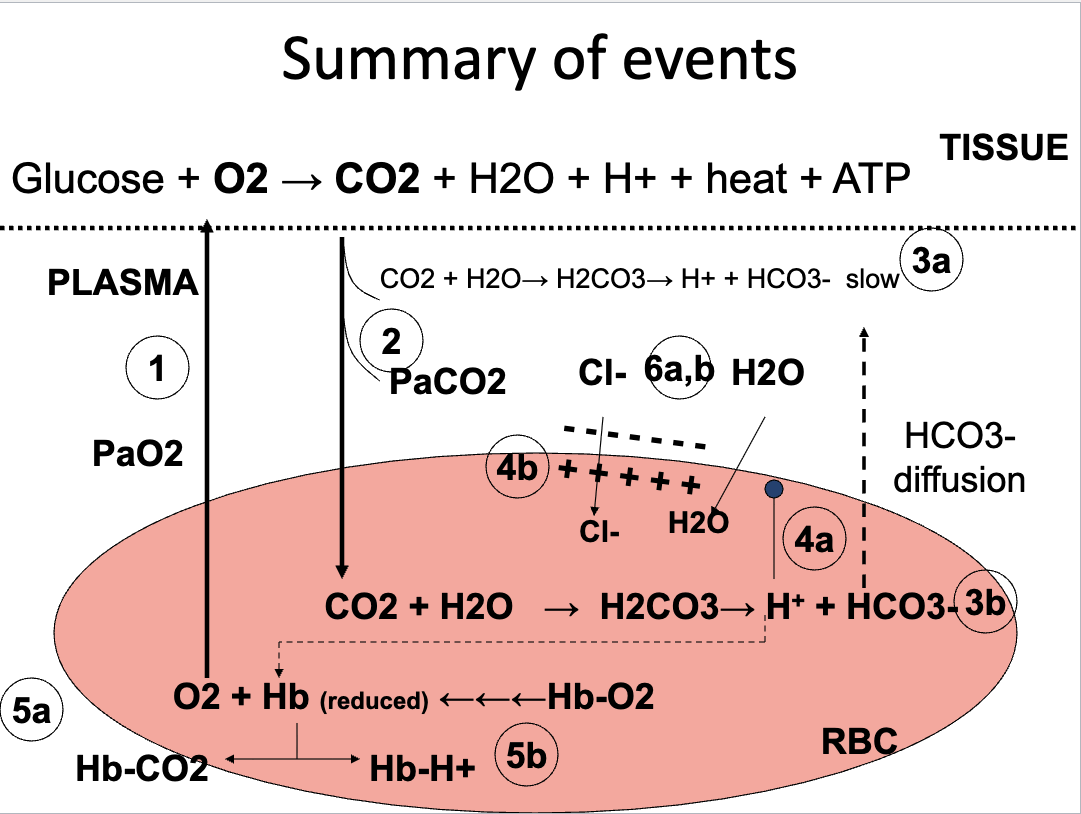
CO2 from blood to lung
My partial pressure in the alveoli is 40, and my partial pressure here is in the blood is 45. When CO2 starts to move down its pressure gradient and leave the red blood cell, that's when all of these things start to get reversed.
Bicarb is a huge blood buffer, hydrogen ions play a huge role in pH, and we… when we blow off CO2, in a way, we are also removing hydrogen ions, right? Because we're binding them up with water, and this is what's being blown out.
And being removed as a waste product. So, respiration is also part of making